| Pages:
1
2
3 |
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Yes, it certainly is, but you could also get the equivalent mass at the same time by measuring hom much NaOH you needed.
|
|
|
ordenblitz
Hazard to Others
  
Posts: 259
Registered: 18-7-2004
Location: Northwest
Member Is Offline
Mood: Bohr'd
|
|
Received a sample of the unknown substance today from 12AX7.
0.007 grams of the roughly ground crystal were mixed with 0.118 grams of powdered IR grade Kbr and gently ground in an agate mortar and pestle. This
sample was then loaded into a McCarthy NAB die set and compressed until it remained only slightly opaque. This die set was then loaded into the beam
path of a Nicolet 360 FTIR and analyzed.
The library search called it: MgSO4*7H2O however the comparison entry was tested in a nujol mull and not in Kbr tablet. So there were some slight
differences in the spectra due to the different sampling method. I then prepared another sample die, this time with commercial magnesium sulfate. The
second spectrum was compared to the first and they were so identical the second spectra looked like a shadow of the first.
So here Tim has Epsom salt of at least USP grade or better. Good guessing Unionized.
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
What do you mean "guessing"? 
BTW, a few tens of ppm of lead or copper would put it outside the USP specification and I doubt that would show up on the IR so I don't think you can
sell it as USP. On the other hand, I'm sure it makes a good drying agent if you bake it for a while.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Thanks!
Now a wider question...why am I getting Epsom Salts from the basement masonry, and why did I observe that 5g dehydrated to 0.7g? (I'll check that
again with a larger measure, and do some gravimetrics yet.)
Tim
|
|
|
ordenblitz
Hazard to Others
  
Posts: 259
Registered: 18-7-2004
Location: Northwest
Member Is Offline
Mood: Bohr'd
|
|
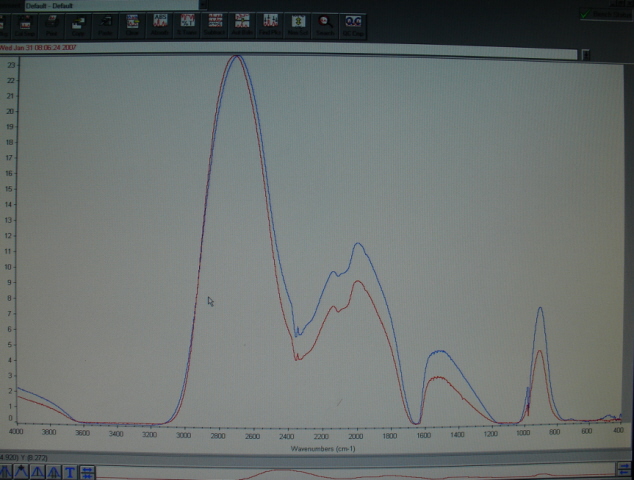
Here is a photo of both spectra on the screen of the FTIR.
The red one is Tim's sample. The blue one is MgSO4*7H20, USP grade.
They don't often come out this close.
[Edited on 31-1-2007 by ordenblitz]
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
"Now a wider question...why am I getting Epsom Salts from the basement masonry, and why did I observe that 5g dehydrated to 0.7g? "
You don't, by any chance live in Epsom do you? OK, i'm kidding but it isn't rare in groundwater- that's how it got a common name.
The stuff usually spits like crazy when I try to dry it
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
That is a pretty nice match, LOL!
If it were a combination salt, such as including sodium or potassium, would those also show up? (I would suppose such inclusion would "distort" it
quite a bit away from the spectra of U.S.P. Epsomite though.)
I mean, the walls the stuff was/is seeping through are dolomite, and I can imagine something like sulfuric acid leeching it out preferrentially (hrm,
is (Ca,Mg)CO3 + SO4(2-) <--> MgSO4 + ... preferred, I mean wouldn't CaSO4 be better?), but I don't really know where the sulfate comes from. I
would be prone to expect chloride instead, due to what road salt is used around here (depending on time of year of course). We aren't in a
particularly acidic rain region.
Tim
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
I should have mentioned that I also get MgSO4 in the efflorescence from the (un plastered) walls in my roof space (the roof has a few leaks).
To me that srongly sugests that it's from the bricks or cement. SO2 from a coal fired kiln might be a sulphur source here.
IR is not good at detecting low level impurities in materials. In some cases it's completely blind. Those IR spectra have no contribution from the N2
and O2 that the beam passed through.
[Edited on 1-2-2007 by unionised]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
On a much larger sample (of somewhat less purity), I got a much more believable water loss. It wasn't heated as hot, though.
A pie tin was weighed as 20g (give or take 1.25g on all measurements). Loaded with the salt, 657.5g, therefore there were 637.5g of salt. This was
melted over direct flame (low) until the consistency was putty-like (reaching a temperature of 214°F / 101.1°C... kitchen digital thermometer  ). It was then cooled and crushed. Total mechanical loss is estimated at 10+/-10g.
The material was baked at 250°F for five hours, then 400°F for two hours. The tin was then weighed at 380g, or 360g dry salt, for a weight loss of
277.5g (257.5 to 277.5g including mechanical loss). Taking the best fit value of 267.5g, this corresponds to 42.0%. If the dry formula is MgSO4,
molecular weight 120, this suggests 166.4 atw H2O, or about 9 hydrate. If the dehydration is indeed 51.3%, then this suggests I lost 33 grams
mechanically, which I doubt. ). It was then cooled and crushed. Total mechanical loss is estimated at 10+/-10g.
The material was baked at 250°F for five hours, then 400°F for two hours. The tin was then weighed at 380g, or 360g dry salt, for a weight loss of
277.5g (257.5 to 277.5g including mechanical loss). Taking the best fit value of 267.5g, this corresponds to 42.0%. If the dry formula is MgSO4,
molecular weight 120, this suggests 166.4 atw H2O, or about 9 hydrate. If the dehydration is indeed 51.3%, then this suggests I lost 33 grams
mechanically, which I doubt.
I don't know where I got 5 > 0.7g before, I might've forgotten a tare weight or something.
So, MgSO4 not within experimental error, at least yet. Next I'll have to actually analyze it chemically.
Tim
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Could there be MgSO4 solution ocluded in imperfections in the crystals?
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
I don't know how much, but I did find it curious that, on breaking a large crystal apart, it glistened with moisture...
Tim
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Given the near perfect IR match, I'd say the water is roughly the equivalent of the difference between a 7 and a 9 hydrate.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Try treating some finely ground material with alcohol, even 70% rubbing alcohol, filter, evaportate, see if anything was dissolved.
Sulfates and neutral phosphates generally have low solubilities in alcohol, Mg and Ca halides and nitrates are reasonably soluble in alcohol.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
I tried drying some ethanol (distilled, maybe 20%, if that) with the stuff I dehydrated. It seems to have turned to a microcrystalline mush.
I also didn't get very good results using sodium sulfate in the same way...
Tim
|
|
|
Unch
Harmless

Posts: 6
Registered: 4-2-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by 12AX7
Thanks!
Now a wider question...why am I getting Epsom Salts from the basement masonry, and why did I observe that 5g dehydrated to 0.7g? (I'll check that
again with a larger measure, and do some gravimetrics yet.)
Tim |
Faith's mysterys.
Damp?Humidity?
[Edited on 12-2-2007 by Unch]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by 12AX7
I tried drying some ethanol (distilled, maybe 20%, if that) with the stuff I dehydrated. It seems to have turned to a microcrystalline mush.
I also didn't get very good results using sodium sulfate in the same way...
Tim |
I was looking more at attempting to see if there was Mg/Ca in excess of SO4, and any Cl or NO3. Combinations of those ions should dissolve in
alcohol, filtering and evaporating the alcohol will show if that had happened.
The weight loss seen is suggestive of either NO3, or of ammonium salts, in addition to MgSO4. Either could be found in water from soil, especially
with roof runoff.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Yeah, but would those crystallize? This is like second or third recrystallization, and I haven't seen crystals of anything else. In fact, less than
50 grams of junk (in a dark yellow to red color, corresponding to the yellow color of the mother liquor) remained after the first crystallization. I
got some blocky crystals from that, probably salt.
Tim
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
I don't know if they would, or form a double salt. I was just suggesting a simple way of checking for minor components without anything fancy. Same
way a quick test for ammonium would be to mix a bit of the mystery stuff with a bit of potassium or sodium hydroxide and a drop of water.
|
|
|
ordenblitz
Hazard to Others
  
Posts: 259
Registered: 18-7-2004
Location: Northwest
Member Is Offline
Mood: Bohr'd
|
|
After receiving the samples, I ground some in preparation of the KBr tablets. It turned into a wet mush from large amounts of occluded moisture in the
crystal. I had to put the sample in an oven at 105c for a short time until dry.
If there were enough impurities to effect the dehydration weight, or any other characteristic different than the pure material, the IR would most
certainly see it. Modern FTIR equipment is usually only fooled by PPM quantity impurity or less.
The absence of peaks from nitrogen and oxygen is because the little effects they have on this short beam path instrument are eliminated by taking a
background before the actual test. What is visible however are the small peaks for CO2 and H2O vapor. I don't bother to purge the sample chamber and
the software corrections for removal of same are only so effective.
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
The FTIR clearly ignores the great bulk of the sample you ran ie the KBr, do you still stand by this assertion "Modern FTIR equipment is usually only
fooled by PPM quantity impurity or less."
As for "The absence of peaks from nitrogen and oxygen is because the little effects they have on this short beam path instrument are eliminated by
taking a background before the actual test."
No it's because IR spectroscopy is blind to homonuclear diatomics like Cl2, N2 and O2 because they simply don't absorb. It's because there's no change
in the dipole moment when the internuclear distance changes.
Think about it, if the thing spots the 330ppm or so of CO2 in the air why not the 210000ppm of O2?
It cannot be, as you say, the short pathlength and the compenstion by the background scan because those are just as true for the CO2 as for the O2.
Some things are just not very good absorbers so they don't show up well.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Hey unionized, congrats on the 1337 posts. 
I don't think N or Cl or O is going to make a difference, in atomic terms. This isn't a Moseley plot. N2 and O2 aren't going to do much in the IR
spectrum being nonpolar, reasonably high binding energy molecules. And that's fortunate so this doesn't have to be done in a vacuum. However, N as
NO3- or NH4+ or others ought to show up somewhere, if present.
What I'm more concerned about, is the IR response more a matter of the crystal structure or the ions (properties of them, that is) inside it? If the
ions, then you should be able to detect the spectra of impurities or compounds (Na, K, etc.) in addition to the same. If it's the structure, then
surely a compound salt would show a different spectrum, necessarily being a different structure.
Whatever it is, it's pure. As I said, this is the only thing I'm crystallizing.
Say, can you subtract the stock MgSO4 spectrum from the measured plot and evaluate the resulting difference? It oughta work that way.. Looking at
the screenshot, it looks like the only differences are the large peak, which is relatively shorter for the blue curve, and a small peak on the far
right end of the spectrum. The overall shape is certainly close beyond a doubt though.
Tim
[Edited on 2-13-2007 by 12AX7]
|
|
|
ordenblitz
Hazard to Others
  
Posts: 259
Registered: 18-7-2004
Location: Northwest
Member Is Offline
Mood: Bohr'd
|
|
unionised:
My posts were clearly brief explanations to avoid getting into complicated FTIR theory. You may decide that instead of nitpicking each word of my
argument to your assertion that IR is not good at detecting low level impurities and yourself go into the laborious explanation on all things FTIR
which I decided not too. The reason KBr is used is because it is essentially invisible to IR in the useful frequency range as are nujol, NaCl and
certain crystals. So what does this have to do with the accuracy of my machine? This Nicolet is good at seeing impurities, I use it each day to do
just that! If you want Tim to send you some of his sample.. I'm sure he would and then you can do some actual lab work on this issue instead of
armchair analysis of mine.
12AX7:
This machine does have a subtract feature. I think its the 7th button in the upper menu from the left. It will subtract one spectra from the other and
you then can search the library for the result. It's usefulness depends on having a really good library of commercial spectra which are very
expensive. I think I only have some 20,000 in my libraries. I didn’t spend a great deal of time in sample preparation as I was just looking to find
out what it was. If I was going to do trace work I would have done a better job of it so as to be more accurate. The differences in the spectra you
observed were due to auto-gain intensity variances since each KBr tablet is somewhat more or less opaque from variability in compression.
[Edited on 14-2-2007 by ordenblitz]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
all of the alkali metal halides are mostly transparent for the IR spec range, which is why IR optics use them. All my experience with IR has been with
organics, except for the unavoidable CO2 and H2O, so I don't really know how inorganics interact. (OK, I know diamond has a notch in the near IR, the
how SiO2, Al2O3, Si, and Ge look).
From what I do know, MgCl2 would be difficult to detect in MgSO4, as it likely doesn't have strong bands in the normal wavelength range. On the other
hand, nitrate should show if if not masked by a much larger amount of sulfate, same for carbonate.
Am I correct, or completely offbase on this?
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Not important, you are right. A small peak can easilly be hidden by a big one. Some materials only have small peaks so they don't show up.
IR, therefore, cannot reliably detect impurities. For example it did not notice the roughly 95% of the sample ordenblitz looked at because (as he
said) KBr doesn't absorb IR.
A perfect IR match does not prove that the compound is pure. NaCl is a very common material. It's a perfectly plausible impurity in the MgSO4. Does
anyone think a few percent of it it would show up in the IR spectrum?
For the record nujol absorbs IR quite well, albeit in a couple of main bands. I've been using IR to identify things for roughly 20 years so I'm not
just an armchair analyst. While it sometimes shows the impurities I don't bet on it for the reasons I gave earlier.
Ordenblitz if you want to do the subtraction 12AX7 sugested earlier you will need to convert the y axis from %t to absorbance. I'd be very grateful if
you would post the spectrum in absorbance units please.
|
|
|
BeanyBoy
Harmless

Posts: 47
Registered: 23-2-2007
Member Is Offline
Mood: No Mood
|
|
A Source for this Stuff?
Hey Tim,
Did you ever come up with a theory about the source of this precipitate, beyond it maybe just being in the ground water?
Did it seep into the basement predominately from one corner, or side, of the house? The house dates to the 1890s you said... do you know where the
Facility was located in those days?
I'm amusing myself with the notion that a former owner of the home had to make regular and frequent use of Epsom Salts despite its side-effects...
-thinking of beans, always....
|
|
|
| Pages:
1
2
3 |