Hilski
Hazard to Others
  
Posts: 197
Registered: 13-9-2006
Member Is Offline
Mood: No Mood
|
|
Capillary tube for vacuum distillations
I was just wondering if anyone has ever successfully used a capillary tube to stir/agitate the contents of a distillation flask during a vacuum
distillation. I finally broke down and got all the stuff to do proper vacuum distillations except for a hotplate with a stirrer. I'm pretty sure my
homemade mag stirrer, which works very well for most things, will not work for this purpose, I was looking into other options since boiling stones
obviously will not work. I was reading through Zubrek's book today and found this, and was wondering if anyone had ever tried it
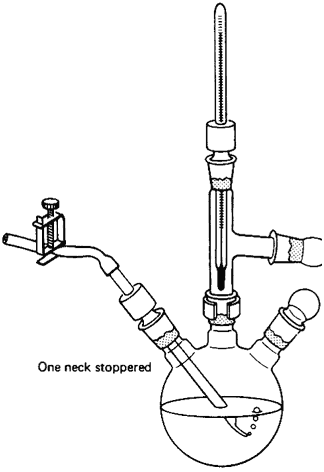
He says in the book that a setup like this is sufficient to prevent bumping during a vacuum distillation. Any opinions?
Thanks
|
|
|
matei
Hazard to Others
  
Posts: 205
Registered: 16-9-2006
Member Is Offline
Mood: No Mood
|
|
Yes, it works. I've done vacuum distillations using capillaries tens of times probably.
|
|
|
nightflight
Hazard to Self
 
Posts: 82
Registered: 23-5-2006
Member Is Offline
Mood: pyrophoric
|
|
A source of air in or just above the reactionfluid works, a.f.a. I can confirm.
|
|
|
unionised
International Hazard
    
Posts: 5124
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Yes, It works. I used a nitrogen stream rather than air because
1 I had nitrogen on tap and
2 My employers are paranoid about safety and they didn't want hot reactive organic vapour and air in the same place at the same time.
Often, the sorts of things you want to vacuum distill are fairly reactive so N2 might not be a bad idea if you can do it.
|
|
|
Hilski
Hazard to Others
  
Posts: 197
Registered: 13-9-2006
Member Is Offline
Mood: No Mood
|
|
Thanks for the replies.
Benzaldehyde is near the top of the short list of things I would like to try and vac distill, and I guess it is prone to oxidation higher temps.
Hopefully, the tiny stream of bubbles allowed in through the capillary tube from the area outside the distillation flask won't cause too many problems
of that nature.
But nitrogen would definitely be great to have. The are dozens of 6000 PSI tanks of nitrogen at any given time where I work, and I doubt that anyone
would really care if I took a little. But I don't have any way to store it, or get it home in the first place for that matter. I do have a few large
fire extinguishers at home that I could probably modify to use for storing nitrogen, but I'm not sure if they are large enough to be of any real use.
In your experience, how large a volume of gas (nitrogen) is needed to run a vacuum distillation for say, 1 - 2 hours?
|
|
|
unionised
International Hazard
    
Posts: 5124
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Just for a start, connecting a fire extinguisher to a 6000PSI tank would almost certainly burst it.
I never meaured the flow rate but it was a few small bubbles per second measured at low pressure so I think a balloon full of N2 would do the job.
Come to think of it, a helium baloon might be a good solution to the problem.
|
|
|
Hilski
Hazard to Others
  
Posts: 197
Registered: 13-9-2006
Member Is Offline
Mood: No Mood
|
|
Wow. I would have thought that it would need a lot more than what a balloon could hold, so I'm glad I asked. You can get helium anywhere, so I may
try that first instead of nitrogen.
And just to clarify: I would definitely use a pressure regulator on the high pressure nitrogen bottle to drop it to a few hundred PSI before hooking
it up to a fire extinguisher bottle. Come to think of it, there probably aren't very many tanks at all that would hold up very well under 6000 PSI.
Thanks
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
Sorry, but the He gas sold for balloons and such is not pure He. The manufacturers put a large amount of oxygen in it so people who inhale it to do
the old funny voice trick don't accidentally asphyxiate.
Try looking for a welding supply store or in the welding section of a hardware store. You can usually find small cheap tanks of inert cover gases for
MIG welding
|
|
|
Hilski
Hazard to Others
  
Posts: 197
Registered: 13-9-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by neutrino
Sorry, but the He gas sold for balloons and such is not pure He. The manufacturers put a large amount of oxygen in it so people who inhale it to do
the old funny voice trick don't accidentally asphyxiate.
Try looking for a welding supply store or in the welding section of a hardware store. You can usually find small cheap tanks of inert cover gases for
MIG welding |
Thanks
|
|
|
unionised
International Hazard
    
Posts: 5124
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
"Sorry, but the He gas sold for balloons and such is not pure He. The manufacturers put a large amount of oxygen in it so people who inhale it to do
the old funny voice trick don't accidentally asphyxiate."
Are you sure about that? The safety data sheet here
http://www.bocballoongas.co.uk/
strongly suggests that, while there may be some air in there, it's not much and the stuff is an axphixiant.
For example the given value for the density relative to air is practiclly that of pure He.
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
It seems that this manufacturer only adds a small amount of air to their He. I expect this to vary from brand to brand.
In any case, when choosing an inert gas for organic work, remember that balloon He is not pure.
|
|
|
unionised
International Hazard
    
Posts: 5124
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
If all else fails you can use CO2 for most things or scrub O2 from air.
It's interesting that I have heard that balloon gas contains hydrogen (to make it cheaper) and air to avoid asphixia, but I've never seen any real
evidence- it's always been a "friend of a friend" sort of thig.
Has anyone ever checked?
|
|
|
Hilski
Hazard to Others
  
Posts: 197
Registered: 13-9-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | | If all else fails you can use CO2 for most things or scrub O2 from air |
CO2 would probably work for most anything I would need to vacuum distill. Enough CO2 to fill a balloon could easily be made with vinegar and sodium
bicarbonate. I suppose the CO2 should be dry and pure enough to use after running through a tube of CaCl2 shouldn't it?
I know O2 can be removed from air with heated copper in a tube, but are there more practical ways to do this?
[Edited on 4-12-2006 by Hilski]
|
|
|
matei
Hazard to Others
  
Posts: 205
Registered: 16-9-2006
Member Is Offline
Mood: No Mood
|
|
You don't need inert gas for distilling benzaldehyde. You can heat the flask with a hand held bunsen burner and the distillation of a small quantity
of benzaldehyde (50 ml or such) takes only a few minutes. You have to wear safety goggles though!!!
| Quote: | Originally posted by Hilski
Hopefully, the tiny stream of bubbles allowed in through the capillary tube from the area outside the distillation flask won't cause too many problems
of that nature.
|
You're right here.
In my laboratory I've never seen anyone use inert gas for vacuum distillations (well, we use it only for organometallics or very air-sensitive
substances).
[Edited on 4-12-2006 by matei]
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Benzaldehyde can be distilled at atmospheric pressure without any problems. The distillate is colorless and free of benzoic acid.
|
|
|
Hilski
Hazard to Others
  
Posts: 197
Registered: 13-9-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | | In my laboratory I've never seen anyone use inert gas for vacuum distillations (well, we use it only for organometallics or very air-sensitive
substances). |
Well, thats what I wasn't really sure about. Benzaldehyde is oxidized fairly easily by air, especially at higher temps. And since I would be
bubbling air right through the hot benzaldehyde, I was slightly worried about losses.
| Quote: | | You can heat the flask with a hand held Bunsen burner and the distillation of a small quantity of benzaldehyde (50 ml or such) takes only a few
minutes. |
Yet another part of the concern. I'm talking about distilling multiple liters, as opposed to smaller amounts which would perhaps distill fast enough
to where I wouldn't have to really worry about it having time to be oxidized. (Well, I'll probably only do 1 liter at a time, but you get the
point.)
|
|
|
matei
Hazard to Others
  
Posts: 205
Registered: 16-9-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Hilski
I'm talking about distilling multiple liters, as opposed to smaller amounts which would perhaps distill fast enough to where I wouldn't have to really
worry about it having time to be oxidized. |
In that case it will be a good idea to use inert gas.
BTW benzaldehide has a very pungent smell - be careful when you handle large quantities.
[Edited on 4-12-2006 by matei]
[Edited on 4-12-2006 by matei]
|
|
|
matei
Hazard to Others
  
Posts: 205
Registered: 16-9-2006
Member Is Offline
Mood: No Mood
|
|
The best inert gas is argon because it is heavier than air and has a "blanketing effect". Argon is easy to find I guess because it is used for inert
gas welding as neutrino said.
[Edited on 4-12-2006 by matei]
|
|
|
Hilski
Hazard to Others
  
Posts: 197
Registered: 13-9-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by garage chemist
Benzaldehyde can be distilled at atmospheric pressure without any problems. The distillate is colorless and free of benzoic acid.
|
I thought it would start to decompose at its boiling point at atmospheric pressure. (178.1)
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
I've been pulling bleeder tubes every other day when I gotta do vacuum distillations.
What I wonder though is what is the exact mechanism that helps with bumping? It's not agitation as the tube is not lead to the bottom of the liquid,
but more likely on the very surface.
 
|
|
|
zed
International Hazard
    
Posts: 2281
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Yeah, seems like I remember doing that. Just heat up a section of a piece of glass tubing, and pull it out into a very, very fine filament. Like a
hair. Under vacuum, enough gas will be pulled through "the hair" to "nucleate" the distillation process. The flow of gas through the tiny filament
is further constrained and controlled by a clamp on the latex tube that feeds it.
I always just used a system, that was fed by the ambient atmosphere.
If you need inert gas... A balloon or two, full of CO2 would do for most purposes. Provided CO2, won't screw up your distillate. I suppose N2 would
be even better. Just depends. Argon is also widely available. More available than Nitrogen actually.
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Sometimes pulling the tube can be a bit tricky, it melts too fast and the capillary will be very short, and sometimes you basically form a glass fiber
of half a meter long. It is easy to cut that fiber to proper length. I'm not sure how fine it can be, but what I do know is that it is easily too
large. A fine appearing capillary can blast havoc in the flask, so the human hair analogue should be taken seriously when referring to what size is
good. I haven't used restrictor tubes though.
|
|
|
Mateo_swe
National Hazard
   
Posts: 532
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
Im looking to buy a TIG welding unit and when exploring different welders i noticed many gases are available on ebay for delivery right to the door.
There are argon, helium, CO2, nitrogen, oxygen and they are about 120Euros + 5Euro shipping for a 8liter, 150Bar cylinder including regulator.
No Hydrogen cylinders on Europe ebay unfortunately but they can probably be bought at a welding supply company.
|
|
|