Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
HNO3
This is a precise description of the method obtaining concentrated HNO3 by ammonium nitrate and H2SO4 within several steps. I`ve packed all files into
a archive and i hope it can be downloaded correct. The file contains a HTML document and any small images. The document will have some errors, i hope
it will corrected and that will told to me.
Enjoy !
[Edited on 13-7-2006 by Mason_Grand_ANNdrews]
Attachment: HNO3.zip (397kB)
This file has been downloaded 512 times
|
|
|
Darkblade48
Hazard to Others
  
Posts: 411
Registered: 27-3-2005
Location: Canada
Member Is Offline
Mood: No Mood
|
|
I'm slightly wary of downloading and open files that are in exe format (blame the recent influx of spyware/spam/viruses/trojans). Perhaps you can
reupload the file in a zip file with the HTML document as well as the images.
|
|
|
Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
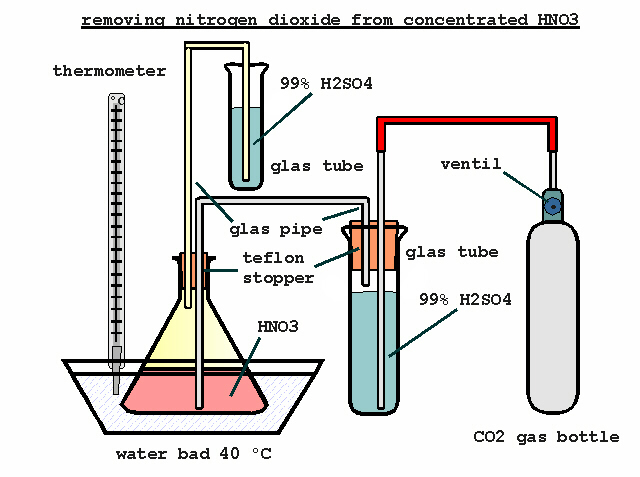
You are right, i`ve uploaded the file in zip format. The next attachment will show a simple method removing nitrogen dioxide from concentrated HNO3.
It will give a way to seal the condenser against moisture. Concentrated H2SO4 is a oily liquid and this will be suitable for small amounts. 98% H2SO4
will it do just as well.
|
|
|
Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
I`ve a little hint to the CO2 gas which is cool when it will be dryed in the H2SO4 tube. Put the tube in the water bad or the tube is heated separate.
A slight stream of CO2 will be sufficient. The CO2 gas is slight heated with water in a dimroth cooler, this will do the same.
[Edited on 15-7-2006 by Mason_Grand_ANNdrews]
|
|
|
Polverone
Now celebrating 21 years of madness
        
Posts: 3186
Registered: 19-5-2002
Location: The Sunny Pacific Northwest
Member Is Offline
Mood: Waiting for spring
|
|
Sorry I didn't see this earlier. Can you update your text with the additional illustration and perhaps some explanatory text? Then, if it is okay with
you, I will make some slight revisions to the text and turn the document into a PDF that will go into the collection of member publications. I think
HTML is fine, but as a PDF the text and all illustrations are combined into a single file, and it can be read immediately unlike the .zip.
PGP Key and corresponding e-mail address
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
The information above concerning the synthesis of HNO3
from ammonium nitrate and sulfuric acid is inaccurate .
The proportions and temperatures are incorrect .
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
I agree. 300 deg C is nowhere near required to achieve full distillation of HNO3. Full decomposition is the only achievement of this experiment.
Furthermore, using CO2 to expel NOx from HNO3 seems rather tedious, considering that urea, or urea nitrate are fully sufficient to do the same job in
highly concentrated (>98% or so) HNO3, which should have been distilled in the absence of UV (sunlight) or high temp - meaning that the HNO3 is
yellow at the most to start off with. If your HNO3 is fuming and red, then something was done wrong.
Mason - we appreciate enthusiasm - the Prepublication section could use some more input undoubtedly- but enthusiasm has little worth without hard
backup. You described the destillation very well, I loved the pictures - but obvious discrepancies with all known observations tend to make the
non-layman doubt everything else. I'd suggest you review your article. Possibly post pictures, and cite references. This isn't a club where one can
combine internet pages and make one grand page out of it. Nothing ends up here without hard backup. And there is simply no way you'd end up with 99%
HNO3 by distilling up to 300 deg C.
PS yields are also not stated as 'ml' but rather as percentages. 100 ml means nothing if you had H2O influx, evaporation, and so on. It is weight per
volume, which essentially describes the density, and thus the percentage of HNO3, which is what we are after (and not the theoretical yield of the
reaction - which is close to 100% anyway).
[Edited on 16-7-2006 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
Thats to bad that the topic not stand more in Prepublication . 
My descriptions are not constituted by a lot of internet documents. I think the desciption will work, distillation 70% HNO3 below 300 °C.
bp of 98% H2SO4 < 338 °C
bp of AN < 302 °C
I will make a small HTML to the theme removing NO2 from HNO3.
|
|
|
chromium
Hazard to Others
  
Posts: 284
Registered: 27-6-2005
Member Is Offline
Mood: reactive
|
|
I think you should do some test runs with these methods, and make measurements for concentration, yield, temperature and other parameters which may be
of interest to others. If such experimental data are collected then there should not be too lot of work to remake this for good (pre)publication.
[Edited on 18-7-2006 by chromium]
|
|
|
Mason_Grand_ANNdrews
Hazard to Self
 
Posts: 63
Registered: 19-1-2006
Location: New Berlin City !!
Member Is Offline
Mood: crabby
|
|
I´ve remaked the HTML document and packed the files into a zip arcive. The document includes the point removing NO2 from HNO3.
Attachment: HNO3.zip (547kB)
This file has been downloaded 462 times
|
|
|