| Pages:
1
2
3 |
OneEyedPyro
Hazard to Others
  
Posts: 280
Registered: 7-10-2015
Member Is Offline
Mood: No Mood
|
|
A high explosive will continue to sustain its own detonation indefinitely so long as it is at what's called critical diameter. If you filled a 30
centimeter wide pipe with ammonal it could be set off with a single small blasting cap and regardless of how long that pipe is the detonation would
sustain itself.
Sugar has an oxygen balance of -112, ammonium nitrate is +20. So it would take about 18 grams of sugar per 100 grams of ammonium nitrate to achieve a
neutral OB. You want about an 85/15 ratio of AN to sugar, just as Gawain said.
You can use copper tubing pinched or hammered shut at one end in place of ammo casings, just don't go squeezing or hammering the other end once it's
got ETN inside.
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 167
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Weeblordd  | | Why not? You mean to cover my ears? Idk about that, I'd prefer not to (I wanna experience it in all its brilliance) |
What I meant was, don't listen to those people who say things like "start with small amounts and then move on to bigger ones", "the first blast
doesn't have to be big", and stuff like that. And above I actually gave as an example that I myself started with a few kilograms, and nothing bad
happened. Ammonium nitrate explosives are very safe, there is no reason to be afraid of making them in large quantities.
In fact, in a domestic setting, pure ETN will be more powerful than PETN, as ETN can be melted, while PETN will require a hydraulic press to achieve
high density. So even though PETN is more powerful at 150-200 m/s, ETN can be poured and pressed more easily, which is preferable for most people.
The benefit of PETN is high yield with low acid consumption. For 200 ml of sulfuric acid you can make 50-60 g of ETN (70% nitric acid method), or 220
g of PETN.
Guanidine derivatives have poor energy content and explosion temperature. The same dinitroguanidine is at the level of HMX in terms of density and
detonation velocity. But in practice it shows itself at the level of less powerful RDX.
[Edited on 31-1-2024 by DennyDevHE77]
|
|
|
Weeblordd
Harmless

Posts: 20
Registered: 6-8-2018
Member Is Offline
Mood: high power <3
|
|
Great idea, and damn I mistook sucrose for glucose lol, my bad, but interesting. How come? Sucrose is C12H22O11, yes. Now, 12(+4) + 22(+1) + 6(-2), 48
+ 22 + (-12) = 58 - 12 = 46, or is it -46? won't matter in the end. So sucrose a pyro valence of -46. Ammonium nitrate has a pyro valence of -2. So in
the end. 46NH4NO3 + 2C12H22O11 = 25N2 + 13H2O + 24CO2. Please share your thoughts, I just started learning this stuff.
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 470
Registered: 12-10-2022
Location: [REDACTED]
Member Is Offline
Mood: Stable
|
|
I’m not exactly sure how you are doing it, but the way I calculate oxygen balance is to find how much oxygen the oxidizer can provide,
(NH4NO3 = N2 + 2H2O + O, 1 mole of oxygen per mole of AN) and how much oxygen the fuel needs to convert it
to water and carbon dioxide, (C12H22O11 = 11H2O + 12C, needs 24 moles of oxygen to combust one mole of
sucrose) and divide the second number (24) by the first (1), and that’s how many moles (24) of oxidizer you need per one mole of fuel. By weight
it’s 24•80=1920g AN and 1•342=342g sucrose, or 85% AN and 15% sucrose.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
Energetics-testin
Hazard to Self
 
Posts: 53
Registered: 7-6-2022
Location: Canada,Quebec
Member Is Offline
Mood: in love with EM's <3
|
|
Quote: Originally posted by OneEyedPyro  |
You seem determined to use thermal shock to detonate your ETN. In order to get that working reliably you want a rapid application of heat such as
flash or black powder and strong confinement, not a slow heating confined in aluminum foil which is liable to give deflagration or partial detonation.
|
i totally forgot to mention what was the mixture used in my detonators, my fault.
I sometimes use other mixtures, but the one I use most is a traditional slow burning black powder with the ratio of 60,30,10.
To help with the transfer of heat to the main charge, "Stuffing" the detonator in a copper tube should help to keep the main charge from being
overheated by the pyro mix before detonating.
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 167
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Weeblordd, don't worry so much about a detailed oxygen balance (ratio) calculation. All fuel components for ammonium nitrate explosives can be
conditionally divided into resinous substances or aluminum (the most powerful, breezability according to Hess 15-16 mm), fiber, sugars and everything
else (medium, breezability about 13 mm), and hydrocarbon fuels (coal, diesel oil, paraffins, breezability 9-10 mm). By comparison, bulk TNT has 13 mm.
Take components either as in historically used mixtures, or +- in stoichiometric ratio.
More importantly, the maximum detonation velocity of 3,800 to 4,000 m/s is achieved when these explosives are loaded into holes drilled in rock, with
a diameter of about 150 mm or when exploding in narrower (100 mm) steel casings with a wall thickness of several mm. At diameters close to critical
detonation velocity of 1500-1700 m/s is realized. In addition, for such explosives, from oxidizer and propellant, it is very important for uniform
grinding of components, so that the decomposition products of some components do not prevail over others. If this happens, you increase the length of
the shock wave front (the zone of chemical reactions increases), chemical losses of individual components increase, which has a bad effect on the
detonation capability of the finished explosive (the tendency to deflagration, explosive combustion, increases). In simple words: power drops
significantly, and some of the explosive often fails to react
|
|
|
Weeblordd
Harmless

Posts: 20
Registered: 6-8-2018
Member Is Offline
Mood: high power <3
|
|
Quote: Originally posted by Sir_Gawain  | | I’m not exactly sure how you are doing it, but the way I calculate oxygen balance is to find how much oxygen the oxidizer can provide,
(NH4NO3 = N2 + 2H2O + O, 1 mole of oxygen per mole of AN) and how much oxygen the fuel needs to convert it
to water and carbon dioxide, (C12H22O11 = 11H2O + 12C, needs 24 moles of oxygen to combust one mole of
sucrose) and divide the second number (24) by the first (1), and that’s how many moles (24) of oxidizer you need per one mole of fuel. By weight
it’s 24•80=1920g AN and 1•342=342g sucrose, or 85% AN and 15% sucrose. |
Surprisingly I haven't yet heard of your method, the way I tried calculating things was explained in this video and also in this article. Thanks for the explanation though, helps a lot
Quote: Originally posted by Energetics-testin  |
To help with the transfer of heat to the main charge, "Stuffing" the detonator in a copper tube should help to keep the main charge from being
overheated by the pyro mix before detonating. |
Great idea, I hope I can put my main detonator inside a bigger strong paper tube also made of regular paper and A4 glue, perhaps may work even better
than a copper pipe to make sure the pyro mix doesn't ignite the ammonal charge
Quote: Originally posted by DennyDevHE77  | Weeblordd, don't worry so much about a detailed oxygen balance (ratio) calculation. All fuel components for ammonium nitrate explosives can be
conditionally divided into resinous substances or aluminum (the most powerful, breezability according to Hess 15-16 mm), fiber, sugars and everything
else (medium, breezability about 13 mm), and hydrocarbon fuels (coal, diesel oil, paraffins, breezability 9-10 mm). By comparison, bulk TNT has 13 mm.
Take components either as in historically used mixtures, or +- in stoichiometric ratio.
More importantly, the maximum detonation velocity of 3,800 to 4,000 m/s is achieved when these explosives are loaded into holes drilled in rock, with
a diameter of about 150 mm or when exploding in narrower (100 mm) steel casings with a wall thickness of several mm. At diameters close to critical
detonation velocity of 1500-1700 m/s is realized. In addition, for such explosives, from oxidizer and propellant, it is very important for uniform
grinding of components, so that the decomposition products of some components do not prevail over others. If this happens, you increase the length of
the shock wave front (the zone of chemical reactions increases), chemical losses of individual components increase, which has a bad effect on the
detonation capability of the finished explosive (the tendency to deflagration, explosive combustion, increases). In simple words: power drops
significantly, and some of the explosive often fails to react |
Big thanks, that's very useful information, so whenever people detonate ammonal charges attached to things like trees or metal plates, etc., only
1500-1700m/s VOD is reached, damn prob would have never realized. May I ask, what is breezability and hess? haven't heard of these terms before
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 470
Registered: 12-10-2022
Location: [REDACTED]
Member Is Offline
Mood: Stable
|
|
I think he meant brisance, and the Hess test is a measurement of how much a certain explosive flattens a lead cylinder.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
OneEyedPyro
Hazard to Others
  
Posts: 280
Registered: 7-10-2015
Member Is Offline
Mood: No Mood
|
|
If you take -1600 and divide it by the molar mass of a substance then multiply it by the remaining surplus of fuel or oxygen you get the oxygen
balance. For example, with methanol -1600 divided by its molar mass of 32 gives us a value of -50. CH3OH has 3 carbon, 4 hydrogen and 1 oxygen, since
it takes 2 oxygen to oxidize a carbon we give it twice the value, it take 1 oxygen to oxidize 2 hydrogen atoms giving it a value of 0.5.
So (-1600/32=-50) x (6C+2H-1O=7) -50x-7 gives us an oxygen balance of -350.
|
|
|
averageaussie
Hazard to Self
 
Posts: 92
Registered: 30-4-2023
Location: Right behind you
Member Is Offline
Mood: school 
|
|
Am I misunderstanding what you are saying here?
that aside, please be careful with detonating even a very small amount of explosive, it can be extremely dangerous as many others have pointed out.
Ammonal has a detonation velocity of about 4,400 meters per second [1], giving it an RE factor of about 0.75. this means you 2kg of it would be
equivalent to 1.5 kg of tnt. conveniently, there is a study that uses exactly this amount, and it found that at a distance of 18 meters, injuries
would still be sustained [2].
you are also using 500 grams of ANSU, which there is sadly little information on, at least none I could find. using ANFO as a rough comparison, your
500 grams is equivalent to about 400 grams of tnt, making up a total of nearly 2kg of tnt equivalent in total.
considering that you say in your original post that this would be your first detonation of a HE, this is, as we say in the industry, a terrible idea.
sources:
[1] https://en.wikipedia.org/wiki/Ammonal
[2] https://www.researchgate.net/figure/Effects-generated-when-a...
|
|
|
Weeblordd
Harmless

Posts: 20
Registered: 6-8-2018
Member Is Offline
Mood: high power <3
|
|
Quote: Originally posted by OneEyedPyro  | Sugar has an oxygen balance of -112, ammonium nitrate is +20. So it would take about 18 grams of sugar per 100 grams of ammonium nitrate to achieve a
neutral OB. You want about an 85/15 ratio of AN to sugar, just as Gawain said.
|
Just out of curiosity, could you share your math behind this? Is this it (below)?
Quote: Originally posted by OneEyedPyro  | If you take -1600 and divide it by the molar mass of a substance then multiply it by the remaining surplus of fuel or oxygen you get the oxygen
balance. For example, with methanol -1600 divided by its molar mass of 32 gives us a value of -50. CH3OH has 3 carbon, 4 hydrogen and 1 oxygen, since
it takes 2 oxygen to oxidize a carbon we give it twice the value, it take 1 oxygen to oxidize 2 hydrogen atoms giving it a value of 0.5.
So (-1600/32=-50) x (6C+2H-1O=7) -50x-7 gives us an oxygen balance of -350. |
And also, @DennyDevHE77, thanks for the info as well, y'all included
Quote: Originally posted by averageaussie  |
Am I misunderstanding what you are saying here?
Ammonal has a detonation velocity of about 4,400 meters per second [1], giving it an RE factor of about 0.75.
|
To clarify, slowly but steadily I've been studying primarily only low explosives, not high explosives. When I said "I just started learning this
stuff", I was referring to learning the math behind explosive design & engineering. Also, pretty please share the math behind the correlation
between R.E. factor and velocity of detonation, sadly the 4.4km/s can only be achieved in a confined setting, when unconfined, only 1.5-1.7km/s. And
also sorry I misspoke back there, I said it would be my first time ever detonating a HE and it's gonna be 2kg ammonal + 500g ansu. To correct myself
- it will be my first detonation of a secondary high explosive, I'll be testing ETN numerous times, uh, on second thought ETN is both a primary and a
secondary so nvm
[Edited on 2-2-2024 by Weeblordd]
[Edited on 2-2-2024 by Weeblordd]
|
|
|
OneEyedPyro
Hazard to Others
  
Posts: 280
Registered: 7-10-2015
Member Is Offline
Mood: No Mood
|
|
Sucrose has a molar mass of 342.3 and its formula is C12 H22 O11.
So, (-1600/342.3=-4.674) x (C24+H11-O11=24) -4.674x24 gives us -112.176.
Ammonium nitrate has a molar mass of 80 and its formula is NH4NO3.
So, (-1600/80=-20) x (2H-3O=1) this gives us -20 but since it's a surplus of oxygen that's actually +20.
So, (+20x85=+1700) (-112x15=-1680) 85/15 AN sugar is nearly perfectly oxygen balanced.
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 470
Registered: 12-10-2022
Location: [REDACTED]
Member Is Offline
Mood: Stable
|
|
Were is the -1600 number coming from? I feel like I'm missing something obvious...
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
dettoo456
Hazard to Others
  
Posts: 250
Registered: 12-9-2021
Member Is Offline
|
|
That formula (and the 1600 as a constant) is used for determining OB as a %. It’s the formula on Wikipedia (https://en.wikipedia.org//wiki/Oxygen_balance) and is generally referred to for most EMs in literature.
You can work backwards from the derived OB% to form EM formulations depending on the desired OB%.
The method laid out in Dugan’s video is generally the same procedure but it’s a little more convoluted since you need to assign values to the
atoms based on their valences, whereas the other method is quicker but requires MW.
Here is an automatic calculator in case anyone wants it - it’s from Klapotke’s lab: https://emto.eu/software/ob-calculator/
|
|
|
averageaussie
Hazard to Self
 
Posts: 92
Registered: 30-4-2023
Location: Right behind you
Member Is Offline
Mood: school 
|
|
Quote: Originally posted by Weeblordd  | Also, pretty please share the math behind the correlation between R.E. factor and velocity of detonation, sadly the 4.4km/s can only be achieved in a
confined setting, when unconfined, only 1.5-1.7km/s.
|
getting 0.75 re factor from 4.4km/s is based on the similar but slightly slower ANFO from the tnt equivalent page, in the table about RE factor.
https://en.wikipedia.org/wiki/TNT_equivalent#Relative_effect...
and the calculator here
https://www.omnicalculator.com/physics/tnt-equivalent
for a det speed of 1.5 to 1.7, it would be an RE factor of about 0.15 if I am correct.
Thank you for clearing up my confusions.
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 167
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Weeblordd  | | Big thanks, that's very useful information, so whenever people detonate ammonal charges attached to things like trees or metal plates, etc., only
1500-1700m/s VOD is reached, damn prob would have never realized. May I ask, what is breezability and hess? haven't heard of these terms before
|
Well, in the final analysis, not all mixed ammonia explosives behave this way, somewhere it is enough to increase the critical diameter a little so
that it becomes optimal, the same ammotropine, as a homogeneous mixture (cast melt), albeit crushed, still has a much closer contact between the
combustible and oxidizer particles, and the requirements for the critical and optimal detonation diameter are much less. Mixtures such as aluminum,
machine oil, and ammonium nitrate are also often used; such mixtures detonate at 120-150 mm case with velocities of 4 km/s. Well and a big plus, they
do not dust unlike just a mixture of aluminum and ammonium nitrate.
For example:
Granulite A-6 PA NH4NO3 - 90%, diesel fuel - 4%, aluminum - 6% Heat of explosion 1100 kcal/kg. Volume of explosion products 870l/kg. Fugacity
400-410ml. Detonation velocity 4200-5000m/s at density 0.95g/cm3 (Charge in a cardboard shell 150mm in diameter).detonation diameter is 100-120mm in a
paper shell and 25-30mm in a steel shell.Minimum initiating impulse 5-10g TNT.
You see, the charge has widened by a few cm and the speed has increased to maximum. Note that this is a granulite, that is, a granular mixture. I
suppose in the form of a fine powder, it will be no worse, maybe even better.
About the Hesse test. It used to be a popular breezability test for explosives. Brizancy is not precisely defined, it varies from country to country,
there was even a big controversy where the argument was caused by the fact that the opponents understood different things by this term. But here by
breezability is meant the ability to directly destroy the nearest medium in close contact with the explosive charge (usually 1.5 times the diameter of
the charge). The Hess test takes a lead cylinder, puts a steel plate on it, and detonates a charge of explosive, 25g (PETN/RDX) for high explosives,
50g for medium explosives (TNT, TNP), and 100g for low explosives, the charge is taken at a density of 1.0 g/mL. Then measure how much the lead column
has shortened. In mm.
I think Laboratory of Liptakov had a video with the tests of Hess, he threw them here on the forum not so long ago
But of course this test, as well as the Kast test and the sand test. They're all obsolete. Now they use the steel plate method. An explosive charge is
detonated on several steel plates, and an ultrasonic scanner measures the resulting cavity.
[Edited on 2-2-2024 by DennyDevHE77]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1419
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
Brizance by Hess..... https://www.youtube.com/watch?v=dxJLIk7dStw
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
Weeblordd
Harmless

Posts: 20
Registered: 6-8-2018
Member Is Offline
Mood: high power <3
|
|
Quote: Originally posted by OneEyedPyro  | Sucrose has a molar mass of 342.3 and its formula is C12 H22 O11.
So, (-1600/342.3=-4.674) x (C24+H11-O11=24) -4.674x24 gives us -112.176.
Ammonium nitrate has a molar mass of 80 and its formula is NH4NO3.
So, (-1600/80=-20) x (2H-3O=1) this gives us -20 but since it's a surplus of oxygen that's actually +20.
So, (+20x85=+1700) (-112x15=-1680) 85/15 AN sugar is nearly perfectly oxygen balanced. |
Thank you for sharing the math. The simpler the better
well ammonal getting a R.E. factor of 0.15 is rather low but sorry my bad, to reach 4.4km/s for VOD for ammonal it's more about critical diameter than
confinement but then again, many factors are at play as you know.
Quote: Originally posted by DennyDevHE77  |
Granulite A-6 PA NH4NO3 - 90%, diesel fuel - 4%, aluminum - 6% Heat of explosion 1100 kcal/kg. Volume of explosion products 870l/kg. Fugacity
400-410ml. Detonation velocity 4200-5000m/s at density 0.95g/cm3 (Charge in a cardboard shell 150mm in diameter).detonation diameter is 100-120mm in a
paper shell and 25-30mm in a steel shell.Minimum initiating impulse 5-10g TNT.
You see, the charge has widened by a few cm and the speed has increased to maximum. Note that this is a granulite, that is, a granular mixture. I
suppose in the form of a fine powder, it will be no worse, maybe even better.
|
yeah, very interesting, I also think powder form would be superior, hopefully. Also, that is an amazing mixture because it seems to be very powerful
for being extremely cheap, do you know any other cheap as hell AN-based explosives?
Cool to see a HESS test in action, thx for sharing.
|
|
|
Nemo_Tenetur
Harmless

Posts: 31
Registered: 13-12-2023
Location: Germany
Member Is Offline
|
|
The critical diameter seems to be a complex phenomenom, at least in such Ammonal mixtures. For my understanding a close to oxygen balanced mixture
should have the smallest critical diameter, but the reality seems to be different.
Zygmunt et. al. performed and published some tests in 2009. To my surprise, with as little as 5 percent aluminum the smallest critical diameter was
achieved, well below 10 mm, whereas a close to oxygen balanced mixture (about 18 percent aluminum) was almost the double value, about 15 mm.
I remember that the same happens with the sensitivity of such mixtures ( with 5 % aluminum considerably more sensitive than with 18 percent),
although detonation velocity and explosive strength goes on par with oxygen balance - why ?
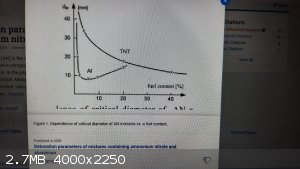
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 167
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
The same phenomenon is observed in ANFO mixtures. at 3% of the diesel fuel, the critical diameter is 30 mm, at 6% (oxygen balanced) it is already 40
mm.
The explanation is that ammonium nitrate decomposes first, and with a diesel fuel content of 6%, it wraps the ammonium nitrate particles too tightly,
preventing them from decomposing quickly.
I suppose the same thing happens with mixtures of aluminum and ammonium nitrate.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1419
Registered: 2-9-2014
Location: Tel Aviv University
Member Is Offline
Mood: old jew
|
|
Let's not forget that ammonium nitrate is explosive itself as pure. AN is not a binary mixture oxidizer + fuel. Therefore ratio for example on 80%
causes lower VoD. And bigger critical diameter. In this ratio the fuel works a like inert material. Works a like diluting of AN. Therefore is
advantager using high reactive fuel with high negative OB. For example wax OB - 345. AN 94,5 + WAX 5,5 = OB - 0.075. Oil has also OB - 345.
Highest power is possible is usually combination more fuels to 10% maximaly.....
Development of primarily - secondary substances: CHP (2015) neutral CHP and Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024) Diper
60 (2025)
|
|
|
OneEyedPyro
Hazard to Others
  
Posts: 280
Registered: 7-10-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nemo_Tenetur  | The critical diameter seems to be a complex phenomenom, at least in such Ammonal mixtures. For my understanding a close to oxygen balanced mixture
should have the smallest critical diameter, but the reality seems to be different.
Zygmunt et. al. performed and published some tests in 2009. To my surprise, with as little as 5 percent aluminum the smallest critical diameter was
achieved, well below 10 mm, whereas a close to oxygen balanced mixture (about 18 percent aluminum) was almost the double value, about 15 mm.
I remember that the same happens with the sensitivity of such mixtures ( with 5 % aluminum considerably more sensitive than with 18 percent),
although detonation velocity and explosive strength goes on par with oxygen balance - why ?
|
The main driving force in the detonation of ammonium nitrate mixtures using a powdered non energetic fuel is the decomposition of the ammonium nitrate
itself, not the oxidation of the fuel component which largely occurs behind the shock front.
Aluminum powder does a good job of sensitizing AN even at low percentages but as the ratio of Al goes up the ammonium nitrate particles become not
only further from each other, but the Al exhibits more and more a cushioning effect between the AN particles.
That's my theory anyway.
I'm fairly certain that the VoD and brisance is similar if not lower at some point as Al content rises, but the heaving capability and the final
energy output is obviously greater as OB gets closer to 0.
With liquid fuels I assume more fuel is oxidized within and very close to the shock front having a positive effect on VoD and brisance, especially
with fuels that dissolve ammonium nitrate.
[Edited on 5-2-2024 by OneEyedPyro]
|
|
|
Weeblordd
Harmless

Posts: 20
Registered: 6-8-2018
Member Is Offline
Mood: high power <3
|
|
Great ideas. What's more powerful though, or loudest, rather - "granulite A-6 PA" or "ammonal 80/20"?
Well, I decided to use thermal shock to detonate around 0.5g unpressed ETN which would detonate 10g of melt-cast ETN, which will detonate 95/5 ammonal
which will detonate 90/10 ammonal and Granulite A-6 PA.
P.S. I have 2.5kg ammonium nitrate in total. So for the main charge I decided to dedicate:
20% of my ammonium nitrate to ammonal 95/5.
40% of my ammonium nitrate to 90/10 ammonal.
And the remaining 40% to Granulite A-6.
And on top of this 1-2kg sugar. For a thermobaric effect. Sound decent?
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 470
Registered: 12-10-2022
Location: [REDACTED]
Member Is Offline
Mood: Stable
|
|
Sounds like one heck of a bang!
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
DennyDevHE77
Hazard to Others
  
Posts: 167
Registered: 15-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Weeblordd  | | Well, I decided to use thermal shock to detonate around 0.5g unpressed ETN which would detonate 10g of melt-cast ETN, which will detonate 95/5 ammonal
which will detonate 90/10 ammonal and Granulite A-6 PA. |
A completely meaningless set of intermediates. 10g of ETN in cast form will be enough above the roof. Don't complicate what you don't have to
complicate.
But the sugar at the top is most likely just sprayed. And the effect of sugar will be completely minuscule, of course, in comparison with real
thermobaric compounds. In general, thermobaric mixtures are usually a mixture of metallic fuels with volatile nitroesters.
For example:
Ammonium perchlorate – 30%, ethyl nitrate – 18%, aluminum – 50%, thickener
(polybutadiene rubber) – 1%, Synthetic fatty acids – 1%. The TNT
equivalent in explosion pressure is 2.0.
Or here is the composition of the contents of secondary explosives (more powerful).
RDX or HMX – 15%, ethyl nitrate – 40%, aluminum - 38%, thickener
(polybutadiene rubber) – 7%. TNT equivalent in terms of explosion pressure 2.3.
Aluminum is interchangeable with zirconium.
|
|
|
| Pages:
1
2
3 |
|