ludemas19
Harmless

Posts: 4
Registered: 15-1-2024
Member Is Offline
|
|
Manganese cleanup and purplish smokes
After performing some reactions with permanganate (manganese eptoxide, thunderstorm in test tube and similar) I was undoubtedly left with a bunch of
nasty brown-tinted glassware. For cleaning it, I found that a dilute H2SO4 + H2O2 mixture works quite well (just a few seconds of soaking and the
glassware is good to go!). So as to give some numbers: dissolved ca 5 mL of sulfuric acid in 500 mL of water and added 100-ish mL of 20-30% peroxide
solution. My best guess is that manganese is being reduced to the colorless and soluble +II state, in the acidic medium, by the peroxide. Upon further
research, I came across the fact that manganese dioxide actually acts as a catalyst in hydrogen peroxide decomposition, therefore it shouldn't really
be consumed. I think that it's probably the acidic medium that changes the game and the presence of sulfate in solution probably complexates the
Mn(II) formed and doesn't allow a re-oxidation. Do you have some more details on this mechanism? About what stops manganese to close the catalytic
cycle?
On another note, I've discovered that, upon quenching with some water, spent manganese eptoxide liberates a purplish-smoke. I wonder what that is: I'd
say it's droplets of unreacted permanganate solution, but I'm not sure. Pretty much like in this video: https://youtu.be/5BCrsIetZoY?si=zXj6kbCbyuMOHOze Any ideas?
[Edited on 29-1-2024 by ludemas19]
|
|
|
Precipitates
Hazard to Others
  
Posts: 131
Registered: 4-12-2023
Location: SE Asia
Member Is Offline
Mood: Acid hungry
|
|
I recently did some experiments with manganese heptoxide - reactions of alcohol and cotton balls - so despite my limited chemistry knowledge will give
this answer a go! I also used manganese dioxide to test hydrogen peroxide concentrations a long long time ago!!
A) Sulphuric acid reacts with hydrogen peroxide to form peroxymonosulfuric acid (1), the main component of “piranha solution”, which will then
react with manganese dioxide to form manganese sulphate (2):
1) H2SO4 + H2O2 → H2SO5 + H2O
2) H2SO5 + MnO2 -> MnSO4 + H2O + O2 ?
Given the quantities involved there is likely to be a large excess of hydrogen peroxide, but with all of the manganese dioxide already converted to
manganese sulphate, there is no catalysis of its decomposition. This theory can be tested by adding more manganese dioxide to the solution, which
should immediately start to decompose the hydrogen peroxide, as long as all of the sulphuric acid (peroxymonosulfuric acid) is depleted.
B) Yeah, probably permanganate in the vapours emanating from the heated sulphuric acid solution. In the video you can see the vapours are darker
(manganese dioxide) when the mixture reacts (violent decomposition of manganese heptoxide probably due to the increased heat of the solution), as
compared to the normal vapours being produced by the mixture.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
This gives me nightmare flashbacks of the time I learned that Mn2O7 could ignite dichloromethane, and the work it took to clean the fume hood
afterwards.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Cleaning fresh MnO2 stains off glassware can be done easily with HCl.
Your mix has a lot of H2O2. A lot more than is needed.
In this setup the peroxide is acting as a reducing agent, which is a bit unusual. I don't think there is anything much happening with the sulfate ion.
Acidified KMnO4 solution + peroxide is a classic disappearing act. You guessed correctly that the manganese ends up an Mn(ii) and is soluble. I don-t
think I could talk confidently about the mechanism.
IMO, hydrogen peroxide is easy to underestimate - for something otc, it is actually quite potent. Especially at high concentrations. Ditto for
permanganate. Once I learned what it is capable of, I was surprised that it is so readily available. By all means experiment. But it is prudent to
keep quantities and/or concentrations small.
|
|
|
bnull
Hazard to Others
  
Posts: 435
Registered: 15-1-2024
Location: South of the border, wherever the border is.
Member Is Online
Mood: Dazed and confused.
|
|
A 3% solution of peroxide with some drops of sulfuric or hydrochloric acid does the job quite well. All you need is some free H+ in the
solution.
As for the mechanism, I don't believe in the formation of peroxymonosulfuric acid, at least not in a solution with less than 1% of sulfuric acid and
6% peroxide.
Quote: Originally posted by ludemas19  | | My best guess is that manganese is being reduced to the colorless and soluble +II state, in the acidic medium, by the peroxide. Upon further research,
I came across the fact that manganese dioxide actually acts as a catalyst in hydrogen peroxide decomposition, therefore it shouldn't really be
consumed. I think that it's probably the acidic medium that changes the game and the presence of sulfate in solution probably complexates the Mn(II)
formed and doesn't allow a re-oxidation. Do you have some more details on this mechanism? About what stops manganese to close the catalytic cycle?
|
Spot on. The sulfate is not essential because any anion that doesn't precipitate Mn2+ would work. The mecanism is bit more complex because
it involves Mn+2 and Mn+3, and possibly HOO- and O2-. The simplified version is the following:
the surface of the manganese dioxide is reduced to Mn2+ with liberation of oxigen:
MnO2 + H2O2 +2H+ => Mn2 + 2H2O + O2.
In contact with water it forms Mn(OH)2, which is insoluble (and forms a layer over the MnO2 underneath), and H+:
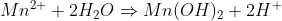
Finally, the Mn(OH)2 is oxidized by the peroxide to regenerate the MnO2 that you had in the beginning.
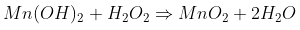
If the solution is acidic, the equilibrium in the first equation is shifted to the right. The Mn2+ is solubilized and the cycle is broken
(either because the Mn2+ goes directly into the solution or the Mn(OH)2 is formed and immediately attacked by the acid; maybe
both, who knows). And the funny part is that the peroxide acts both as reducing and oxidizing agent in the same solution.
The purple smoke may be residues of permanganate, a mist of permanganic acid (which is violet, if I'm not mistaken) made airborne by the reaction, or
both.
Quod scripsi, scripsi.
B. N. Ull
P.S.: Did you know that we have a Library?
|
|
|
Precipitates
Hazard to Others
  
Posts: 131
Registered: 4-12-2023
Location: SE Asia
Member Is Offline
Mood: Acid hungry
|
|
Quote: Originally posted by j_sum1  | | IMO, hydrogen peroxide is easy to underestimate - for something otc, it is actually quite potent. Especially at high concentrations. Ditto for
permanganate. Once I learned what it is capable of, I was surprised that it is so readily available. By all means experiment. But it is prudent to
keep quantities and/or concentrations small. |
Definitely. I forgot about a plastic bottle (as supplied) of 30% H2O2 I had in a wooden cabinet at my parents house in the UK, and ended up leaving it
for several years without checking it. When I went back last month, the H2O2 had eaten through the plastic (now cracked and brittle) and several
centimetres of wood at the bottom of the cabinet. Needless to say, this now illegal chemical has disposed of itself! The wood was bleached white and
totally destroyed. Luckily it was an old cheap cabinet in the undecorated utility room (concrete floor), so no real damage done.
|
|
|
ludemas19
Harmless

Posts: 4
Registered: 15-1-2024
Member Is Offline
|
|
Quote: Originally posted by j_sum1  |
IMO, hydrogen peroxide is easy to underestimate - for something otc, it is actually quite potent. Especially at high concentrations. Ditto for
permanganate. Once I learned what it is capable of, I was surprised that it is so readily available. By all means experiment. But it is prudent to
keep quantities and/or concentrations small. |
Absolutely, I was quite surprised by how reactive both were and the fact that they are easily available makes me shiver a bit. That H2O2 I used (30%)
was bought by my school in a simple local hardware store: which, yeah, is a bit scary. Of course, I always try to pay attention and work with
concentrations as low as possible. (woops, probably I exaggerated with the peroxide in that cleanup bath, but nothing bad happened fortunately, just a
lot of foaming)
|
|
|
ludemas19
Harmless

Posts: 4
Registered: 15-1-2024
Member Is Offline
|
|
Quote: Originally posted by bnull  |
Spot on. The sulfate is not essential because any anion that doesn't precipitate Mn2+ would work. The mecanism is bit more complex because
it involves Mn+2 and Mn+3, and possibly HOO- and O2-. The simplified version is the following:
the surface of the manganese dioxide is reduced to Mn2+ with liberation of oxigen:
MnO2 + H2O2 +2H+ => Mn2 + 2H2O + O2.
In contact with water it forms Mn(OH)2, which is insoluble (and forms a layer over the MnO2 underneath), and H+:
Finally, the Mn(OH)2 is oxidized by the peroxide to regenerate the MnO2 that you had in the beginning.
If the solution is acidic, the equilibrium in the first equation is shifted to the right. The Mn2+ is solubilized and the cycle is broken
(either because the Mn2+ goes directly into the solution or the Mn(OH)2 is formed and immediately attacked by the acid; maybe
both, who knows). And the funny part is that the peroxide acts both as reducing and oxidizing agent in the same solution.
The purple smoke may be residues of permanganate, a mist of permanganic acid (which is violet, if I'm not mistaken) made airborne by the reaction, or
both. |
Thank you very much for the answers! Makes a lot of sense that the acid stops the catalytic cycle. I guess the catalysis is done in neutral or basic
conditions iirc. The fact that peroxide acts as both reducing and oxidising agent is quite interesting indeed. I thought about using it to convert
iron from III to II (and/or back), to make a project about the chemistry of iron, but if that is the case, I feel I'll have to study up some
procedures about that.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Peroxide will readily oxidise Fe(ii) to Fe(iii).
|
|
|
woelen
Super Administrator
        
Posts: 8013
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
It indeed is quite remarkable that H2O2 can act as a reductor, and also can act as an oxidizer. I think that it works through the intermediate step of
formation of peroxo-complexes.
If you have a solution of MnSO4 and add some dilute NaOH, then you get a pale white/brown precipitate of Mn(OH)2, which is oxidized easily, even by
air. Adding a small amount of H2O2 to this leads to instant oxidation to dark brown hydrous Mn2O3/MnO2. Here H2O2 acts as oxidizer. Any excess H2O2 is
decomposed catalytically to water and oxygen.
If you add some H2O2 to an acidified suspension of Mn2O3/MnO2, then instantly, the maganese is reduced to Mn(2+), which goes into solution. The H2O2
acts as reductor, with oxygen being the product of the reaction. Any acid does the job, even dilute acetic acid.
Other metals also allow reaction of H2O2 as oxidizer, or as reductor.
At high pH, when you add H2O2 to a solution of freshly precipitated Cr(OH)3, or a green solution of chromite, then with some gentle heating, you can
oxidize chromium(III) to chromium(VI). You get yellow chromate. This reaction is not complete and fast, like the reaction with Mn(II) and H2O2, but it
does occur.
On the other hand, if you add H2O2 to an acidified solution of a dichromate or CrO3, then you get an instant reaction, A peroxo-complex of Cr(VI) is
formed: CrO(O2)2. This complex has a deep blue/indigo color. It quickly shows an internal redox reaction though. The peroxo-ligands acts as reductors
on the chromium(VI) kernel and in the process, some acid is consumed. The end product is chromium(III), which goes into solution, oxygen and water.
So, the intensely blue/indigo colored solution of CrO(O2)2 loses its color in minutes and becomes much lighter green or purplish grey, depending on
what anionic species are present (e.g. chloride leads to formation of a green chloro-complex of chromiuM(III), while nitrate does not form a complex
and the reaction then leads to formation of the purplish/grey hexaqua complex of chromium(III)). So, with chromium(VI), H2O2 acts as a reductors and
the mechanism behind it is so slow, that you can nicely see it in action in a simple experiment. I think that with manganese such reaction also
occurs, but that in that case, the complex formation and subsequent internal redox reaction are very very fast (milliseconds or even faster).
With vanadium(V), similar reactions can occur. Peroxide can oxidize vanadium(IV) to vanadium(V), but on the other hand, vanadium(V) can form peroxo
complexes (yellow or blue, depending on concentration and pH), which very slowly (much slower even than with chromium) partially decompose to yield
vandium(IV)-species.
IIRC, another chemical, which has similar mixed redox properties is hydroxylamine. This can act as reductor (with N2 being the end product), but it
can also act as oxidizer (with NH3 being the end product).
[Edited on 31-1-24 by woelen]
|
|
|
ludemas19
Harmless

Posts: 4
Registered: 15-1-2024
Member Is Offline
|
|
That's very cool! tysm for the suggestions!
I've already heard about the chromium peroxo-complex and looked really gorgeous. I'd love to get some chromium and try it in the lab; also some
vanadium too, for its spectrum of colours.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
The chromium complex is very soluble and amazingly stable in organic solvents such as tert-amyl alcohol. It's a blue flash in aqueous solution, but
lasts for several minutes in the alcohol. Obviously, it must be a tertiary alcohol, as it will oxidize a primary or secondary one. I've been
planning to test to see if it lasts in butanone or ethyl acetate (but I suspect the acid would hydrolyze the latter),
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
|