Diachrynic
Hazard to Others
  
Posts: 226
Registered: 23-9-2017
Location: western spiral arm of the galaxy
Member Is Offline
Mood: zenosyne
|
|
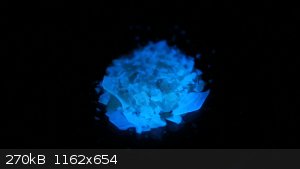
Phosphorescence from boric acid + salicylic acid
Boric acid and a variety of organic compounds[1][3] together can create phosphorescent materials. A particularly accessible example is a
boric acid melt in which 0.1-0.01 % of salicylic acid was mixed in. It shows quite bright blue phosphorescence that lasts for about 10 seconds.
Preparation:
About 5 g of boric acid were mixed with a spatula tip of salicylic acid (aim: about 1-5 mg), placed into a ceramic crucible and melted carefully over
a blowtorch flame. I should note that it is probably a lot better to do this in an oven or by some means of temperature control as my product was
slightly inhomogenous and discolored. The mix releases steam and some boric acid vapor, so ventilation is a good idea. As soon as everything was
melted it was left to cool back to room temperature, then chiseled out of the crucible. Pouring it out did not work, as the viscosity was too high.
The solidified melt behaves like a tough glass.
When hit by a UV light (365 nm), the solid shows bright blue fluorescence. When the light is turned off, a visible blue afterglow is observed that
decays over the course of about 10 seconds (probably depends on how strong the UV lamp was).
I made a video of this here: https://www.youtube.com/watch?v=aqqbfqcU6oE
The crucible could be mostly cleaned by boiling it in water for an hour, although not everything was removed. If the mix was heated for higher and
longer, presumably more would be backed in. A disposable test tube could be a good container for this experiment.
A lot of other compounds also work, including tereophthalic acid and phthalic anhydride, as well as many more, that may also have colors different
from blue. The paper[1] I got this from tested a variety of things.
Finally, I am not toally sure what the mechanism for the phosphorescence here is, but the boric acid/metaboric acid melt forms a glass-like matrix
that restricts molecular rotation and vibration effectively.[2]
Literature:
[1] - E. Tiede, A. Ragoß, Ber. dtsch. Chem. Ges. A/B 1923, 56, 655-666, https://doi.org/10.1002/cber.19230560313
[2] - Z. Wu, J. C. Roldao, F. Rauch, A. Friedrich, M. Ferger, F. Würthner, J. Gierschner, T. B. Marder, Angew. Chem. Int. Ed.
2022, 61, https://doi.org/10.1002/anie.202200599
[3] - Z. Zhang, Y. Shi, Y. Liu, Y. Xing, D. Yi, Z. Wang, D. Yan, Chemical Engineering Journal 2022, 442, 1, 136179, https://doi.org/10.1016/j.cej.2022.136179
[Edited on 24-12-2023 by Diachrynic]
we apologize for the inconvenience
|
|
|
ItalianChemist
Hazard to Others
  
Posts: 172
Registered: 26-1-2011
Location: Italy
Member Is Offline
Mood: No Mood
|
|
Very nice light!
I have tried it in the past, if you add a tiny bit of sodic fluorescein you will obtain a nice green phosphorescence!
|
|
|
walruslover69
Hazard to Others
  
Posts: 234
Registered: 21-12-2017
Member Is Offline
Mood: No Mood
|
|
I worked on Phosphorescence during my PhD research. I spent close to 2 months making boron carbon nitride nanoparticles by pyrolyzing boric acid or
boron oxide with different organic compounds (urea +boric acid worked best) the easiest method was using a microwave. The mechanism for the action of
the phosphorescence is not totally understood, but it is suspected that during pyrolysis a graphene like structure is produced with a significant
number of defects. The unique electron structure of boron (and nitrogen to a lesser extent) can aid in the intersystem crossing of electrons from a
singlet excited sate to a triplet state where phosphorescence occurs. We were able to obtain phosphorescence with emission peaks ranging from
425-530nm depending upon reaction conditions and compounds used. I would need to look back at my notes for the details. Our products were usually off
white to purplish grey colored powders.
Sounds like it might be the same phenomenon but not sure. but hopefully you find it useful. This is a very cool phenomenon that is worth studying. I
remember trying to replicate a paper that produced carbon boron nitride particles with a claimed half-life of 1-2 minutes, and it being visible for 6
minutes. We were never able to come close to this even though the synthesis was extremely simple.
|
|
|
|