Niklas
Hazard to Self
 
Posts: 50
Registered: 1-12-2023
Location: Germany
Member Is Offline
Mood: Polymerized
|
|
Synthesis of iron(III)-acetylacetonate
Greetings everyone, hope you all are having a pleasant advent season.
Today it’s time for me to share an inorganic synthesis I did while renovating my laboratory, which is the synthesis of iron(III)-acetylacetonate
from iron(III)-chloride. Besides it simply forming some really beautiful crystals it may surprise you to hear that actually it‘s also quite a useful
reagent in organic synthesis, since it can be used as a cheap catalyst in alkyl-aryl crosscouplings, replacing precious metals like palladium, which
may not be affordable enough to be used in the average home laboratory.
Unsurprisingly a complex this simple isn’t too hard to synthesize, and it actually just takes two easy steps to produce Fe(acac)3 with reasonably
high yields.
Required chemicals:
-7,5 g of anhydrous iron(III)-chloride
-15 ml of acetylacetone
-some 20% potassium hydroxide solution
-some acetone
To get things started the FeCl3 was dissolved in approximately 100 ml of destilled water with the help of a magnetic stirrer, resulting in a clear,
orangey-brown solution.
Then, to produce the intermediate reactant, 20% potassium hydroxide solution was slowly added to the reaction mix until the pH reached a value of 8.
This reaction should yield ferric oxyhydroxide quantitatively, which, because of its really low solubility in water, could simply be filtered off,
washed with fresh water multiple times and dried inside a vacuum desiccator.
Moving on with the preparation the semi-dry paste was transferred to another beaker, and to start the actual reaction the acetylacetone was simply
added all at once and stirred, so an even suspension was formed. After half an hour the mixture turned a dark red color, indicating that the
conversion was finished.
Once again a simple filtration was performed to collect the product as a red powder, for further purification it was recrystallized from acetone twice
tho, yielding some gorgeous dark-red crystals of iron(III)-acetylacetonate.
Yield: 5,3 g; 32,4% of the theoretical (lost quite a bit of product in the purification, since I really tried to get some good crystals for the shots
under the microscope, quite sure yields of at least 60% can easily be reached too tho)
   
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Very nice!! I always have trouble getting the right solvent for recrystallization of these things.
I've found with the copper(II) acac complex, chloroform seems to work best, although methanol does okay. It's weirdly less soluble in larger
alcohols....
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Niklas
Hazard to Self
 
Posts: 50
Registered: 1-12-2023
Location: Germany
Member Is Offline
Mood: Polymerized
|
|
Good to know, never even thought about using chloroform as a solvent for recrystallizing my Cu(acac)2.
Just tried acetone and methanol before, which worked alright, I just got some really small crystals from it tho..
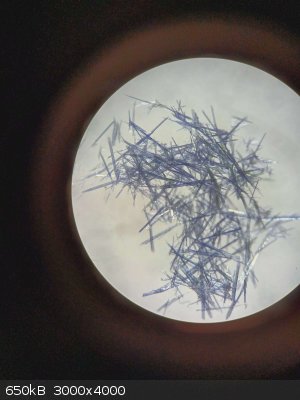
[Edited on 7-12-2023 by Niklas]
|
|
|
walruslover69
Hazard to Others
  
Posts: 234
Registered: 21-12-2017
Member Is Offline
Mood: No Mood
|
|
Fun complex! We synthesized this as well as the copper and cobalt compelx in my undergrad inorganic lab several years ago. It was my first
introduction to FeCl3, my mind was blown when I weighed out the FeCl3 powder, went to the bathroom only to come back and find a toxic rust color
sludge. It absorbed enough moisture from the air to dissolve. Didn't seem to effect the reaction though
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
We use FeCl3 in a Friedels-Craft reaction, and our lab manual reminds the students not to leave it sitting out, and not to breathe near it.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
ItalianChemist
Hazard to Others
  
Posts: 172
Registered: 26-1-2011
Location: Italy
Member Is Offline
Mood: No Mood
|
|
Very nice crystals! Acetylacetone also forms very nice colored complex with many metals!
I love these complexes, since I don't have any acetylacetone I will try with dibenzioylmethane, that should form similar complexes!
[Edited on 9-12-2023 by ItalianChemist]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I've made copper(II) dibenzoylmethanide. It's a green powder, almost completely soluble in everything (although I didn't try chloroform). Very
difficult to clean the residue off the beaker. Resists ammonia and hot concentrated nitric acid.
https://link.springer.com/article/10.1023/A:1009543703278 says its soluble in DCM.
http://www.orgsyn.org/demo.aspx?prep=CV1P0205 says it can be decomposed with sulphuric acid.
I had a paper that claimed it was a monohydrate, but do you think I can find it now?
I also made the nickel(DBM)2, but haven't tried characterizing it yet.
My go-to source for acac syntheses is this:
https://magritek.com/wp-content/uploads/2020/03/Lab-Manual-M...
[Edited on 10-12-2023 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
ItalianChemist
Hazard to Others
  
Posts: 172
Registered: 26-1-2011
Location: Italy
Member Is Offline
Mood: No Mood
|
|
I would like to try with Ru(III) complexes, but unfortunately I can't find much about the preparation of these complexes.
I guess it could be prepared similarly to the Fe(acac)3. I will try it will small amount and see what happens!
|
|
|
Niklas
Hazard to Self
 
Posts: 50
Registered: 1-12-2023
Location: Germany
Member Is Offline
Mood: Polymerized
|
|
There is a patent on the synthesis of different metal acetylacetonates from the corresponding hydroxides, maybe it would help when trying to prepare
the Ru(III) complex.
https://patentimages.storage.googleapis.com/47/cc/41/71fbd1d...
|
|
|
ItalianChemist
Hazard to Others
  
Posts: 172
Registered: 26-1-2011
Location: Italy
Member Is Offline
Mood: No Mood
|
|
Very interesting!Thanks!
I think I will first try the same procedure for the Fe(acac)3,it should be quite similar!
|
|
|
Niklas
Hazard to Self
 
Posts: 50
Registered: 1-12-2023
Location: Germany
Member Is Offline
Mood: Polymerized
|
|
Make sure to do a better job with the purification than me, other than iron ruthenium is expensive xD
Looking forward to see you trying this synth tho
|
|
|
ItalianChemist
Hazard to Others
  
Posts: 172
Registered: 26-1-2011
Location: Italy
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Niklas  | Make sure to do a better job with the purification than me, other than iron ruthenium is expensive xD
Looking forward to see you trying this synth tho |
Ahahah yes but ruthenium has very interesting chemistry!
I will try first with very low quantities to see if it works!
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi Hiklas, some very interesting chemistry reported above and good write-ups. I like the photomicrographs too!
By the way do you have a reference to any of the cross coupling reaction you mentioned in your original post?
|
|
|
Niklas
Hazard to Self
 
Posts: 50
Registered: 1-12-2023
Location: Germany
Member Is Offline
Mood: Polymerized
|
|
Thanks Boffis, nice to hear you like it 
Here is my source for the iron catalyzed alkyl-aryl crosscoupling, planning on trying the reaction out soon by making 9,10-dibutylanthracene:
http://orgsyn.org/demo.aspx?prep=v81p0033
|
|
|