| Pages:
1
2
3
4 |
DrManhattan
Harmless

Posts: 32
Registered: 24-1-2015
Member Is Offline
Mood: No Mood
|
|
I recently made a slurry composition containing by weight: 70% Sodium Chlorate
9% H2O
20% 200 mesh aluminium powder
1% guar gum
Charge was 1kg Detonated with a no.8 blasting cap with 10g ETN booster.
Performance was impressive considering the ease of manufacture and availability of chemicals. Id compare it to anfo in power.
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Storage stability might be an issue with that composition, so you should store the components unmixed.
|
|
|
jpsmith123
National Hazard
   
Posts: 764
Registered: 24-6-2005
Member Is Offline
Mood: No Mood
|
|
IIRC there is a patent from the 60s (Melvin Cook et al.) regarding sodium chlorate based blasting agents using various fuels such as ethylene glycol,
methanol, ethanol and isopropanol.
Unfortunately the patent didn't go into much detail regarding the performance.
|
|
|
DrManhattan
Harmless

Posts: 32
Registered: 24-1-2015
Member Is Offline
Mood: No Mood
|
|
Would storage stability problems arise from the fact that the composition contains aluminium and water? Or am i overlooking something. Id like to find
a way to make it storable.
I came across a page which detailed a few compositions of Sodium Perchlorate mixtures that might be interesting given their stated VOD of 5800-7010
m/s.
Composition #1
67% sodium perchlorate, 33% matrix.
matrix:
Diethylene Glycol 75%
Water 10%
Calcium Nitrate 12.4%
Guar Gum 2.5%
Glacial Acetic Acid 0.1%
Composition #2
60% sodium perchlorate, 40% matrix.
matrix:
61% Aqueous Sodium Perchlorate Solution 30%
Calcium Nitrate 10%
Diethylene Glycol 57%
Guar Gum 2.9%
Glacial Acetic Acid .1%
Composition #3
65% sodium perchlorate, 35% matrix.
matrix:
61% Aqueous Sodium Perchlorate Solution 20%
Calcium Nitrate 10%
Diethylene Glycol 67%
Guar Gum 3%
Glacial Acetic Acid .1%
Composition #4
62% dry sodium perchlorate and 38% matrix.
Matrix:
61% Aqueous Solution of Sodium Perchlorate 25%
Diethylene Glycol 73%
Guar Gum 2%
Composition #5
68% sodium perchlorate 32% matrix.
Matrix:
Diethylene Glycol 74%
Water 11%
Calcium Nitrate 12%
Guar Gum 2%
Glacial Acetic Acid 1%
Composition #6
64.5% sodium perchlorate, 28% matrix.
Matrix:
Diethylene Glycol 84%
Water 12.5%
Guar Gum 2.4%
Glacial Acetic Acid 1.1%
Composition #7
58 sodium perchlorate, 32% matrix.
Matrix:
Diethylene Glycol 75%
Water 11.5%
Calcium Nitrate 13.5%
Composition #8
55% sodium perchlorate, 45% matrix.
matrix:
61% Aqueous Sodium Perchlorate Solution 35%
Calcium Nitrate 10%
Diethylene Glycol 53.5%
Guar Gum 1.5%
"The detonation velocity i listed must have been for all the explosive compositions. So the velocity of these compositions ranges from 5800-7010 m/s
according to the patent. Not just potassium perchlorate and sodium perchlorate can be used, but most inorganic chlorate or perchlorate salts. Some are
more sensitive than others though. 1 lb charges of composition 1 and 2 shattered a 3/4" steel plate and the rest of the compositions blasted holes in
the plate."
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1405
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
I seems that would Alfred Nobel pretty looking on it..
This composition are same power as dynamite 60%. However without dangerous nitroglycerin. Maybe is possible say, that only hygroscopicity is problem
for this mixtures. But it is only one disadvantage. All others properties are better. If you making from this composition dynamite shape and wrapped
in aluminium foil is problem almost nothing. And if is the air around pretty dry, under 50%, is it all better than the dynamite. Produce NaClO4 should
be easily than produce HNO3 + H2SO4 + nitration + neutralisation process.
PS: Mixtures with Al, this type, are stabbile only 24 hrs. Arises Hydrogen.
[Edited on 2-11-2016 by Laboratory of Liptakov]
[Edited on 3-11-2016 by Laboratory of Liptakov]
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
Camroc37
Hazard to Others
  
Posts: 101
Registered: 4-8-2015
Location: USA
Member Is Offline
Mood: Must...Nitrate...EVERYTHING!
|
|
Quote: Originally posted by MadHatter  | Sounds similar to what this thread is describing. One mixture, called rackarock, is made with
potassium chlorate and mononitrobenzene. It was used in New York City to blast out the
Hell Gate Channel on the East River. Right now, I can't find any VODs on this mixture. |
Here's a good demonstration
https://vimeo.com/184011174
|
|
|
DrManhattan
Harmless

Posts: 32
Registered: 24-1-2015
Member Is Offline
Mood: No Mood
|
|
Excellent video. Thanks for the link, it was a shame when explosiopedia's channel was removed from youtube but its good to see that he's active on
vimeo. As for these mixtures containing Aluminium only being stable for 24 hrs due to the production of hydrogen, couldn't you add boric acid to
reduce it? I believe many explosives use boric acid for exactly this reason.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
One could also:
1°)coat the Al dust with HE NC lacker (collodion) and dry...to avoid direct contact of reactive Al with water...the NC would contribute to the
explosive effect.
2°)use perchlorates that are more soluble into organic solvents to avoid water presence.
3°)use solvents or solvaters that dissolves better oxo-salts into organic solvents (quaternary ammonium, crown ethers, cryptants, ...)
4°)Find a composition with concentrated HNO3 that is storage compatible/safe with fuels...
Al is passivated into concentrated HNO3...but unknown is what happens when NaClO4 or other perchlorates are present aswel?
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1405
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
The international situation is very serious. For this reason videos containing energetic materials, and their preparation was be deleted.
Dr. Liptakov
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
@L-Liptakov,
Today I just had the time to watch your two videos on vimeo about Kidonite, a few minutes later they were deleted    ...only remains your fuel C and minium videos (found by searching via Alfred Liptakov). ...only remains your fuel C and minium videos (found by searching via Alfred Liptakov).
The Vimeo Sprengel was stil in action...but for how long?
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
C6(NO2)5CH2CH(CH3)N(NO2)2
Harmless

Posts: 43
Registered: 4-4-2018
Member Is Offline
Mood: No Mood
|
|
I am curious, do you remember if the perchlorate completely dissolved? or were there grains of NaClO4 in the composition after mixing? Also, do you
know how fine the aluminum powder was? Thank you.
Put that in your pipe and smoke it!
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1405
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Of course, was tested yesterday. With a new NaClO4 from acetone separation process. Free available patent: https://patents.google.com/patent/US5665935
...... ...LL ...LL
https://patents.google.com/patent/US5880399A/en
[Edited on 20-2-2019 by Laboratory of Liptakov]
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Has anyone had success with the compositions outside of NaClO4? I made two attempts, first used KClO4 in DOT 3 matrix with water and NaNO3 guar gum
glacial acetic. Second one was embodiment 12 but with NH4ClO4/NaNO3. Interestingly, KClO4 dissolved into solution better than NH4ClO4. Neither one of
my attempts set hard after 24 hrs. Patent claims they should begin setting within a few hours at ambient temp. I mixed them with a paddle stirrer and
attempted to follow the detailed description in patent as closely as possible... Sort of scratching my head.
It would be great to get it to set. There is some fluid separation at top of casts and they are getting more firm, very slowly, but nowhere near the
speed claimed in patent. Id like to figure out which one cures hard then attempt to add some NQ dissolved in DMSO to pump up the numbers a little for
cheap.
|
|
|
EF2000
Hazard to Others
  
Posts: 153
Registered: 10-5-2023
Location: The Steppes
Member Is Offline
Mood: Taste testing the Tonka fuel
|
|
You can try adding acrylic paint.
Quote: Originally posted by Laboratory of Liptakov  | Unfortunately, using calcium nitrate in composition was not tested. Is possible that acetic acid catalyse better properties of guar gum as binder.
But also not tested. Measurement in cavity showing density 1.6 g/cc. Not 1.7. Composition is castable at 80 C without guar gum. Repeatedly.
Composition, respectively his surface is partially hygroscopic. It was observed. Ferocene was not tested. Yes, was tested different acrylic
pigment colors. Yellow, red, green, orange. Interest: This dry (any color) acrylic pigment create
with NH4ClO4 pretty explosive mixture at ratio AP 75 / color 25. Without change of nice color of pigment.
https://www.google.com/search?q=pigment+to+wall+color+paint&...
Maybe colors works as catalyseur of shockwave. (no confirmed, no measured)
But confirmed is better binding effect with acrylic colors. (acryl is binder itself)
Yes, different colors is possible use as identificator.
Else composition can looks: NaClO4 75, DEG 25, liquid pigment (red from link) 11, dH2O 4, guar gum 1. In of part by weight. 20g sensitive on No.8,
confirmed.
[Edited on 10-1-2023 by Laboratory of Liptakov] |
Wroom wroom
"The practice of pouring yourself alcohol from a rocket fuel tank is to be strongly condemned encouraged"
-R-1 User's Guide
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1405
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
NaClO4 75 + DEG 25 is the basis of success. Other mixtures are a lottery bet only. And this despite the fact that they are mentioned in the patents
as functional variations....
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
pjig
Hazard to Others
  
Posts: 179
Registered: 25-5-2010
Member Is Offline
Mood: always learning
|
|
Gotta ask , has there been any success with the original self curing comp? Many attempts have been done with the ammonium perchlorate, and a simi waxy
hard product started to form, but never heated or cured to a firm cast like the patent claims . Wondering if the secret it in the sodium perchlorate.
It’s affinity for water seems like a better candidate for dehydration of the cast , further pushing the nitrate matrix to accelerate the reaction.
To my understanding the finished cast was to be very firm sort of waxy , but not crumbling .
The golden egg is a stable , storable , primer cap sensitive comp/cast.
Ammonium salts where not to this standard, and not cap sensitive.
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
I assume NaClO4 is necessary. I still have a sample of KClO4 and NH4ClO4 comp curing but Ive detected no change in a month. At some point after
testing cure I will try to detonate them in wet state.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1405
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
The key of reactivity NaClO4 +fuel is his a huge solubility in water 209g in 100g H2O at 25C.
Solubility KClO4 is 1.5g in 100g H2O at 25C. (140x less)
Solubility NH4ClO4 is 21g in 100g H2O at 25C. ( 10x less)
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
pjig
Hazard to Others
  
Posts: 179
Registered: 25-5-2010
Member Is Offline
Mood: always learning
|
|
So the lack of soluble oxidizer in solution is the issue. It seems that the answer is the most soluble oxidizing salt. I kinda figured that was was
mostly the issue. I wonder if there is a way to utilize ammonium perchlorate in this function. Also the ammonium perchlorate needs to be dried prior
to adding to the matrix to get accurate weights . The patent claims to use AP as a replacement oxidizer for sodium perchlorate. I’d like to see
success with both. A self vulcanizing effect that can dehydrate and solidify the comp to a #8 cap detonatable material, w/ o use of sensitizing
metals etc.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1405
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
The functional mixture, which is the core of patent and works reliably, is almost always supplemented with 10 other variants and additions of various
substances with which the patented mixture hardly (or at all) no longer works. This is for copyright protection. I will give an example: Man 1)
Ha...!..I claim a patent for a mixture of NaClO4 + DEG in a ratio of 75:25.
Man 2) Ha...!...I use a mixture NaClO4 + DEG 74: 26 and added 5% NH4ClO4 to it. Your patent does not apply to my mixture..!
And this my a new formula mixture works pretty well too..!
In short: Avoid bullshit and gravy when studying patents.... .......(at this
composition you never use Alu powder. Instantly arises small bubbles of hydrogen) .......(at this
composition you never use Alu powder. Instantly arises small bubbles of hydrogen)
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1405
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Due to the questions surrounding the high-performance NaClO4 + DEG mixture, I am attaching pictures. Only 3 substances need to be used for a
successful composition. 1) Relatively pure NaClO4. (not NH4ClO4, not KClO4, no other salt)
2) Diethylene glycol - DEG - (not EG, not TEG, not alcohol, not gasoline, not glycerin)
3) liquid acrylic pigment, US 19.90 https://www.ebay.com/itm/353790837663?epid=7052387860&ha... is used to add color to the wall painting in the apartment. Use green. 10 parts by
weight. So 75 + 25 +10. (do not add more water, guar gum, aluminum, or donkey shit and dried bumblebees)
Procedure: put the 3 components in a closable jar and mix at 20 C. Close and heat in a water bath to 50 C. Open and mix thoroughly, repeatedly. Keep
at 40 - 60 C for 5 minutes. Allow to cool to 20 C. A thick, but partially brittle paste will form, which can be easily filled into the cavity. See
Fig. The mixture has a working name of Greenolite. When heated to 80 C, the mixture can be cast similarly to TNT.
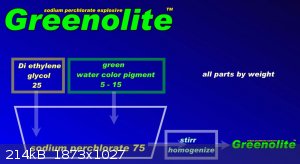  
In cavity by fig. is Greenolite senzitive on No.8. Mixture is partially hygroscopic.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
dettoo456
Hazard to Others
  
Posts: 249
Registered: 12-9-2021
Member Is Offline
|
|
Would there be any way to substitute the DEG for a different polyhydric alcohol or alcohol polymer like polyethylene glycol or PVA? DEG is a little
harder to get ahold of and hygroscopicity is one of the main drawbacks of the composition.
|
|
|
MineMan
International Hazard
    
Posts: 1012
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Laboratory of Liptakov  | Due to the questions surrounding the high-performance NaClO4 + DEG mixture, I am attaching pictures. Only 3 substances need to be used for a
successful composition. 1) Relatively pure NaClO4. (not NH4ClO4, not KClO4, no other salt)
2) Diethylene glycol - DEG - (not EG, not TEG, not alcohol, not gasoline, not glycerin)
3) liquid acrylic pigment, US 19.90 https://www.ebay.com/itm/353790837663?epid=7052387860&ha... is used to add color to the wall painting in the apartment. Use green. 10 parts by
weight. So 75 + 25 +10. (do not add more water, guar gum, aluminum, or donkey shit and dried bumblebees)
Procedure: put the 3 components in a closable jar and mix at 20 C. Close and heat in a water bath to 50 C. Open and mix thoroughly, repeatedly. Keep
at 40 - 60 C for 5 minutes. Allow to cool to 20 C. A thick, but partially brittle paste will form, which can be easily filled into the cavity. See
Fig. The mixture has a working name of Greenolite. When heated to 80 C, the mixture can be cast similarly to TNT.
In cavity by fig. is Greenolite senzitive on No.8. Mixture is partially hygroscopic. |
lol. All true. But I can’t think how aluminum would not help. Or better yet decaborane  ) )
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1405
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
According to indications, some researchers fail to initiate energetic material based on perchlorate aqueous suspension. For this reason, a composition
with as few variables as possible was described, which works reliably. Without aluminum, guar gum, glass microballoons and the like....
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 457
Registered: 12-10-2022
Location: [REDACTED]
Member Is Offline
Mood: Stable
|
|
What’s the point of the green dye? Besides making it look nice, of course.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
| Pages:
1
2
3
4 |