xonu
Harmless

Posts: 14
Registered: 20-5-2019
Location: Ukrainian Soviet Socialist Republic
Member Is Offline
Mood: dissassociating
|
|
Phthalate Metal Complexes
"Recently" I've prepared a Copper Phthalate complex based on a 1967 paper I've found online: https://www.journal.csj.jp/doi/10.1246/bcsj.40.1162
I've also attempted the same procedure with Cobalt (II):
https://isopropyletherperoxide.github.io/2022/06/29/Cobalt.h...
I think they make some very nice colours and are easily accessible to most people and I haven't seen anyone mention them here yet
Oh, and at the moment I'm making more KHP for making the Ni (II) Complex and possibly isolating the anhydrous form of the copper complex (should be a
nice green)
 
[Edited on 29-4-2023 by xonu]
meow
|
|
|
xonu
Harmless

Posts: 14
Registered: 20-5-2019
Location: Ukrainian Soviet Socialist Republic
Member Is Offline
Mood: dissassociating
|
|
Alternatively, the Nickel and Cobalt Phthalates can be made by refluxing their carbonates with Phthalic Anhydride for 6 hours.
However I ended up spilling the bright pink cobalt solution on my bench while evaporating it, and didn't get to see how the crystals look like
https://pubs.acs.org/doi/pdf/10.1021/ja01621a014

[Edited on 29-4-2023 by xonu]
[Edited on 29-4-2023 by xonu]
meow
|
|
|
xonu
Harmless

Posts: 14
Registered: 20-5-2019
Location: Ukrainian Soviet Socialist Republic
Member Is Offline
Mood: dissassociating
|
|
After a while of inactivity, I did end up making the nickel phthalate, which ended up a very pale blue, quite similar to some Cu (II) salts.
 
meow
|
|
|
Fery
International Hazard
    
Posts: 1015
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Well done! The Ni phthalate looks pale green on photos or is it pale blue as you wrote (camera sensors are not so good as human eye)?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
Nice!
I find with various organic acids of the form H2X, I'm never sure if the product I'm getting is Cu(HX)2, CuX, or some form of complex containing
[Cu(HX)X]- or [CuX2]2-. (Not to mention the various hydrates.) Do you have the full paper of your first citation? The full paper's
actually accessible for free! Whoda thunk?
PS: Slava Ukraina!
[Edited on 15-7-2023 by DraconicAcid]
[Edited on 15-7-2023 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
xonu
Harmless

Posts: 14
Registered: 20-5-2019
Location: Ukrainian Soviet Socialist Republic
Member Is Offline
Mood: dissassociating
|
|
Quote: Originally posted by Fery  | | Well done! The Ni phthalate looks pale green on photos or is it pale blue as you wrote (camera sensors are not so good as human eye)?
|
Initially a greenish colour, it turned into a very faint blue, almost white under certain angles after drying and crushing the big clumps of it
You can see the colour quite well in the picture attached next to the other phthalates I've made, and in the other pic where it's between Potassium
Tetrachlorocuprate and Cyanochroite, which I thought were really similar
(also attached is a picture of green bits of it when it was just crystallizing out)
 
[Edited on 12-8-2023 by xonu]
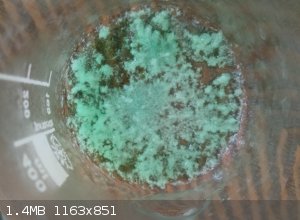
meow
|
|
|