| Pages:
1
2 |
Sulaiman
International Hazard
    
Posts: 3724
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Online
|
|
Control of reflux temperature for fractional distillation - how?
I have not measured this, but I suspect that,
the liquid returned to the top of a fractionating column by the condenser
will be significantly cooler than its b.p.
This must make the top of the column less efficient than it could be.
I have seen diagrams of industrial fractionating columns where
the condensed vapour is returned to the column via a temperature controlled tank.
That makes product take-off easy and maintains fractionating power.
Is there a simple, practical method of doing something equivalent with my NS24 glassware?
PS I'm thinking more of some method of heating the liquid by the up-going vapour
rather than electrically etc.
The simplest alternative is to add more plates,
or just accept non-ideal performance.
[Edited on 13-7-2023 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Lionel Spanner
Hazard to Others
  
Posts: 168
Registered: 14-12-2021
Location: near Barnsley, UK
Member Is Offline
|
|
Industrial fractionating columns are designed for continuous processes; lab-scale equipment is better suited to batch processes, and attempting to
construct continuous-process equipment at that scale is a very complicated task.
In batch processes, the distillate can be manually returned to the heating flask as often as necessary to refine it, and at a lab scale, this is not a
practical problem. Doing this with multiple tons of liquid is a different story, which is why continuous processes, with multiple,
temperature-controlled take-off points, are preferred in industry.
Also, at lab scale, a condenser is more typically attached to the outlet of the column rather than the top, so the mixture is not under reflux.
[Edited on 12-7-2023 by Lionel Spanner]
|
|
|
Rainwater
National Hazard
   
Posts: 938
Registered: 22-12-2021
Member Is Offline
Mood: Break'n glass & kick'n a's
|
|
Quote: Originally posted by Sulaiman  | I have not measured this, but I suspect that,
the liquid returned to the top of a fractionating column by the condenser
will be significantly cooler than its b.p.
This must make the top of the column less efficient than it could be.
[Edited on 12-7-2023 by Sulaiman] |
Assuming you can set up for total reflux and have some control of the takeoff
Then, that statement is true.
While playing with my appratus I noticed that chilled condencer coolant was breaking my equilibrium and causing occillation in my distillate
tempature, The first method tried was is to adjust the flow rate of coolent until about 1/2 of the reflux column was wet. Cheap easy and it worked.
I wanted to do better, adding a thermometer into the coolant return line and adjusted the flow until the coolant returning is about 5-15c below the
target head temperature, resulted in 1/3 to 1/2 of the reflux condencer being wet.
It also saves a lot of ice.
"You can't do that" - challenge accepted
|
|
|
Sulaiman
International Hazard
    
Posts: 3724
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Online
|
|
So far I have been controlling take-off rate (hence reflux ratio)
by varying the cooling water flowrate to the reflux condenser,
allowing some of the vapour to pass through the reflux condenser to the product condenser.
Like this
https://www.sciencemadness.org/whisper/files.php?pid=475345&...
and this
https://homedistiller.org/wiki/index.php/Cooling_management
I can't recommend this method - too sensitive to coolant temperature, and flowrate
requiring frequent tweaking,
but on the positive side, it is easy to implement with common glassware,
and, relevant to this discussion,
the reflux condenser is by design/intent not over-cooling the condensed vapour,
so that's good.
(possibly a reason for tails compression as reported on the homedistiller page pointed to above? )
So, any other suggestions regarding equalisation of the temperature of the liquid returned to the top of the column by the reflux condenser?
I may eventually buy a partial-take-off head,
but this effect will still be of interest to me.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
teodor
National Hazard
   
Posts: 924
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
I did several fractionations with with partial-take-off head and if I put the reflux ration less than 1:5 there is no stable equilibrium in the
column.
Probably you can put something like this part

between the top of the column and the condenser (which you put above, not into the side arm as usuall) and try to regulate the reflux ration just by
regulating the heat (having a good insulation of the system up to the branch point). With the special head you have just a better/faster adjustment,
but I think the partial take-off can work with this simple branch also. Also, I think if you have the condenser above the branch point it should be
enough for not-too volatile liquids and you don't need the second condenser in the side arm.
The key idea is to keep the main condensation point just ~1cm above the branch, not inside the condenser. The liquid which flows down is heated by
vapours and vapours are condensed here because the liquid takes their heat, so the top of the column (where the insulation starts, just below the
branch) will have the temperature almost equal to the boiling point.
You still need the condenser on top, it will create the temperature gradient but will not actually condense the vapours except the most volatile part
of them.
[Edited on 13-7-2023 by teodor]
|
|
|
Sulaiman
International Hazard
    
Posts: 3724
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Online
|
|
I've considered similar
(Vapor Management in home distiller terminology)
but with the addition of some kind of valve
to control reflux ratio.
(eg a disc shaped stirbar/stirdisc with an external magnet)
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Endo
Hazard to Others
  
Posts: 124
Registered: 5-1-2006
Location: USA
Member Is Offline
Mood: Cold
|
|
I have controlled takeoff by using a reflux condenser as a fractionation column, I feed water into the reflux column with a peristaltic pump from a
sink full of cool water. As the temperature at the head increases I just increase the flow through the reflux column a bit to keep it steady and pull
the head temp back down. The reflux ratio changes with each adjustment and by the end of the distillation the takeoff usually decreases.
I know it is not a hands free process, and depending on what is being distilled it can require many adjustments, but for me it works well. I often
struggle to get good separations even with azeotropes because I am at 7300ft elevation.

|
|
|
Sulaiman
International Hazard
    
Posts: 3724
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Online
|
|
Interesting.
Any idea approximately how many theoretical plates this achieves?
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Endo
Hazard to Others
  
Posts: 124
Registered: 5-1-2006
Location: USA
Member Is Offline
Mood: Cold
|
|
Sorry Sulaiman,
If I am consistent babysitting it and maintaining the head temp solidly at a good temp by adjusting the peristaltic pump at a good rate I typically
just capture the fraction I need until the takeoff dwindles to nothing and at that point the higher boiling compounds end up at reflux.
|
|
|
FrecherChemiker
Harmless

Posts: 3
Registered: 10-7-2023
Member Is Offline
Mood: Based
|
|
tbh I have never seen a setup with column + condenser on top of it.
Usually you control the destillation by heating and/or vacuum.
If separation is still not sufficient either use a larger column or distill multiple times.
|
|
|
Rainwater
National Hazard
   
Posts: 938
Registered: 22-12-2021
Member Is Offline
Mood: Break'n glass & kick'n a's
|
|
So today i was forced to disolve and amount of 7,9-Dihydro-1H-purine-2,6,8(3H)-trione in about 8l of water, so while i waited for that, I hobbled over
to my lab and setup a condencer controlled reflux like pictured above.
Pros
It can be built with simple cheap parts most labs have available
Cons
It is not as easy as a variable takeoff head.
The main downside is the difficulty in controlling the reflux, i had better results flowing the coolant backwards through the condencer.
Coolent flow rate was very tricky, but I must say. Once i swapped over from an inline valve to a more controlled wedge valve. I was impressed.
Then a thermometer into the coolant return line really helped.
If you can afford the loss in column length, and the extra time babysitting. This may be a cheaper option than purchasing a variable takeoff head
"You can't do that" - challenge accepted
|
|
|
yobbo II
National Hazard
   
Posts: 765
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
If you are using a hotplate could you use an external temperature sensor attached to condenser top ? You need an external temp. Input on hotplate.
There may be 'hunting' problems. The solution sounds too simple
[Edited on 21-7-2023 by yobbo II]
|
|
|
Rainwater
National Hazard
   
Posts: 938
Registered: 22-12-2021
Member Is Offline
Mood: Break'n glass & kick'n a's
|
|
Ive tried that and having the controlling probe in the distillation head has its pros and cons.
On startup you risk full power being applied to your flask and uncontrolled boiling while the controller tries to figure things out.
The mantel will be adjusting the power for you, so as the composition changes, the boiling points change and the controller is lost at what to do.
In short, you have to move it around alot to get good seperation and fast distillation rates
Without tinkering it will go between maintaining the heat temp without producing distillate, and the distillate tempature oscillating providing poor
seperation.
To start the distillation
1) Place the probe in the boiling flask and slowly increasing the temperature until the solution starts to boil.
2) As the solution gets depleted in volatile components, the distillate will come over less and less until it stops.
3)the mantel power will need to be regularly increased. For that reason, set the temperature controller to the current fraction tempature and move the
controlling probe to the distillate head.
4) the distillate will start coming over but know that it will begin slowing down over time because of the way the controller works. As long as your
not transitioning between fractions, momentarily, turning up the temp, or removing the probe will provide the feedback the controller needs to restore
the distillation rate.
5) when you approach the end of a fraction, the constantly changing head temp will throw the controller into oscillations. Move the probe back into the boiling flask, then adjust the controller temp to the flask current tempature.
6) to finish pushing through a transition keep adjusting the controller until the head temp becomes stable then goto step 3
As far as how this relates to the above constructed appratus, you'll be adjusting 2 sensitive and touchy controls at the same time upping the level of
difficulty. Not saying its. Bad Idea, just staying its going to be a little harder
"You can't do that" - challenge accepted
|
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
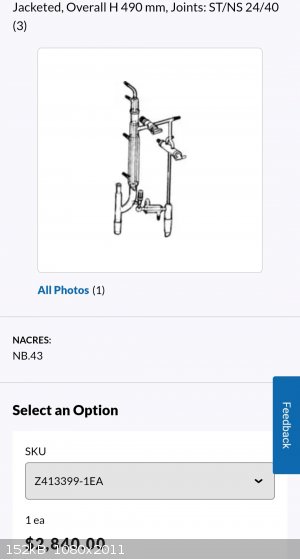
https://www.sigmaaldrich.com/US/en/product/aldrich/z413399
If you're doing a good fractional distillation you need a variable takeoff head to allow return to the column in order to get the proper reflux ratio
and get efficient separation. Even if you have the packing, height and diameter to get 8 theoretical plates if you're just flashing material through
the column with no return you're not going to get all those theoretical plates working as they should. Having been in the industry for awhile this is
the head of choice. Obviously you need the right column for your separation but this gives rock-solid head temperatures reflecting the current
boiling point of whatever component or mixture you're dealing with. This specific head is usually used for vacuum distillation as you can release the
receiver to pressure and swap it out while leaving the pot under vacuum and not having to disrupt the equilibrium in the column.
Anyway, there's a needle valve that adjusts the takeoff rate, you can watch the return rate based on the drips off the condenser. Yes the material is
chilled when dripping off the condenser but the thermometer is in the headspace so it reflects the actual boiling point. This is the setup I would
emulate providing I had a good column.
|
|
|
Sulaiman
International Hazard
    
Posts: 3724
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Online
|
|
That distillation head looks like a rather expensive
'washing up' accident waiting to happen 
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
That was just my lead-in to this one being half the price
https://www.sigmaaldrich.com/US/en/product/aldrich/z544817

Just that without a variable takeoff rate and being able to put the column in steady-state you're not going to be able to achieve maximum separation
with your distillation column.
|
|
|
Rainwater
National Hazard
   
Posts: 938
Registered: 22-12-2021
Member Is Offline
Mood: Break'n glass & kick'n a's
|
|
Agreed but for 2800usd. Ouch. Like that doesnt even fathom in my brain how that could cost that much.
[Edited on 22-7-2023 by Rainwater]
"You can't do that" - challenge accepted
|
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
There's gotta be some Bomex version or something out there though.
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Quote: Originally posted by Sulaiman  | the liquid returned to the top of a fractionating column by the condenser
will be significantly cooler than its b.p. |
The heat of evaporation / condensation is much more higher than specific heat capacity. E.g. for water specific heat capacity 4,186 J/g°C and heat of
evaporation 2256 kJ for 1 kg at 100°C at atm. pressure, so heat of evaporation is more than 500 times higher than specific heat capacity.
The colder reflux liquid is quickly heated to the boiling point at the cost of small amount of vapor condensation. There is always some distance (at
least few cm) between the cooling part of the condenser and the top of the filling in the column which is enough for thermal equalization when
distilling laboratory amounts.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2753
Registered: 26-1-2011
Location: USA - NC
Member Is Online
Mood: Mildly disgruntled scientist
|
|
If people really want some fancy distillation heads, I have a variety. A few are pristine, a few have damage or missing stopcocks that need to be
replaced, and others are just odd. But way cheaper than the ones shown, I am sure. I have all sorts of stills, heads, various DeanStark type
things, and more. I am happy to sell them.
|
|
|
arkoma
Redneck Overlord
      
Posts: 1763
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
With EtOH, from raw sugar wash (estimated 16-18% w/hydrometer), I can get 90% purity easily in one run. I had to learn to apply heat slowly and I use
a 4 litre Erlenmeyer with a 300mm 24/29 air condenser with five "balls" of copper mesh spaced out in it, still head/thermometer up top, 105° adapter
on that going into the top of a 300mm Graham condenser. I ease up the temp and when the vapor front climbs I pay attention and back the temp down a
little. I like to run "low and slow" and keep my fractionating column ALMOST choked down with liquid returning to the flask. It's pretty efficient at
giving me a good fraction, but you better pull up a chair because it ain't fast.
Sulaiman, I remember when your technology for distillation consisted of a long glass tube at an angle with a wet towel draped on it for cooling. Come
a ways!
[Edited on 8-2-2023 by arkoma]
[Edited on 8-2-2023 by arkoma]
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Grizli7
Harmless

Posts: 41
Registered: 1-9-2021
Member Is Offline
|
|
Handbuch der Laboratoriumsdestillation (Krell, 1976) may be this help
|
|
|
Grizli7
Harmless

Posts: 41
Registered: 1-9-2021
Member Is Offline
|
|
https://dl.iranchembook.ir/ebook/General-Chemistry-616.pdf
|
|
|
Sulaiman
International Hazard
    
Posts: 3724
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Online
|
|
Quote: Originally posted by arkoma  | | With EtOH, from raw sugar wash (estimated 16-18% w/hydrometer), I can get 90% purity easily in one run. |
you
might benefit from a quick 'stripping run' (simple distillation) to get 'low wines' (typ. 40+%ABV)
before doing a fractional distillation.
The volume to go in the pot is significantly reduced (to about 1/3rd)
(or three times as much product per tedious fractional distillation)
and foaming during fractionation is almost eliminated.
Grizli7: thanks for the pdf. Concise and useful.
Fery: thanks, I didn't consider the huge ratio,
so the slightly over-cooled condensate from the reflux condenser,
falling on the top of the column,
is not as significant an imperfection as I was imagining..
- still not perfect 
[Edited on 17-12-2023 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Grizli7
Harmless

Posts: 41
Registered: 1-9-2021
Member Is Offline
|
|
5-10max
|
|
|
| Pages:
1
2 |