Parakeet
Hazard to Self
 
Posts: 75
Registered: 22-12-2022
Location: Japan
Member Is Offline
Mood: V (V)
|
|
Liquefying SO2 into a Plastic Bottle
According to this graph, the boiling point of SO2 at 4-5 atm is about 30℃. [Source]
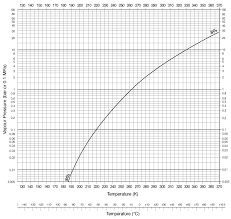
And since 830 kPa of pressure is exerted on normal carbonated water bottle, I thought these carbonated water bottles can safely keep liquefied SO2 at room
temperature, at least in theory.
Could this be an effective cylinder for SO2, or would it be as stupid as it sounds?
|
|
|
Lionel Spanner
Hazard to Others
  
Posts: 168
Registered: 14-12-2021
Location: near Barnsley, UK
Member Is Offline
|
|
It's very likely to dissolve or severely corrode the plastic, especially under pressure.
Also, how would you open it without it vaporising and going everywhere?
|
|
|
teodor
National Hazard
   
Posts: 922
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
If you can it would be great savings of money, because I paid 40 EUR + for 10 atm glass bottle.
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 457
Registered: 12-10-2022
Location: [REDACTED]
Member Is Offline
Mood: Stable
|
|
Yes, but I would rather pay 40 EUR than have a bottle of liquid SO2 explode in my face.
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2799
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Is stainless steel not compatible with liquid SO2?
|
|
|
teodor
National Hazard
   
Posts: 922
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
I have only one high pressure bottle from stainless steel and have no idea where to buy more, so I don't know the price of it.
|
|
|
Sulaiman
International Hazard
    
Posts: 3720
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I've no expertise with liquid SO2 storage,
but I can imagine several problems;
eg flash-boiling if pressure released at RT. (valve required)
or brittle plastic if stored in a freezer (bp=-10oC @ 1atm)
I suspect that a plastic bottle will work fine 
- until it doesn't 
I would rather generate SO2 on demand than store liquid SO2
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 457
Registered: 12-10-2022
Location: [REDACTED]
Member Is Offline
Mood: Stable
|
|
Why would you even want to store SO2? To get around the EU sulfuric acid ban?
[Edited on 1-7-2023 by Sir_Gawain]
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
Parakeet
Hazard to Self
 
Posts: 75
Registered: 22-12-2022
Location: Japan
Member Is Offline
Mood: V (V)
|
|
That might be a problem. I couldn’t find the chemical resistance data of PET against liquid sulfur dioxide. Does anyone have it?
Or I think I should test it by dipping a small piece of PET in liquid SO2, but then I need a pressure resistant container to prepare it…
@Sulaiman, @Sir_Gawain
Yeah, you are actually right. But I still think cylinders are slightly easier to use when you want to use SO2 in reactions : you won't need another
flasks and dropping funnel to generate it.
|
|
|
Parakeet
Hazard to Self
 
Posts: 75
Registered: 22-12-2022
Location: Japan
Member Is Offline
Mood: V (V)
|
|
Oh, and what apparatus did you buy, specifically? Do you have any pictures or a link to the manufacture?
|
|
|
Lionel Spanner
Hazard to Others
  
Posts: 168
Registered: 14-12-2021
Location: near Barnsley, UK
Member Is Offline
|
|
Quote: Originally posted by Parakeet  |
That might be a problem. I couldn’t find the chemical resistance data of PET against liquid sulfur dioxide. Does anyone have it?
|
PET gets knackered by a water/acetone mixture. It's got no chance whatsoever standing up to liquid sulphur dioxide.
|
|
|
Sulaiman
International Hazard
    
Posts: 3720
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Polypropylene (nalgene) looks to be compatible with SO2
Attachment: Graco_ChemCompGuideEN-B.pdf (687kB)
This file has been downloaded 196 times
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Parakeet
Hazard to Self
 
Posts: 75
Registered: 22-12-2022
Location: Japan
Member Is Offline
Mood: V (V)
|
|
Interesting! Nice reference.
Unfortunately though, PP becomes weak in cold places, iirc.
|
|
|
teodor
National Hazard
   
Posts: 922
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
Parakeet, just do search "proglass pressure vessel", you will find the info. The only one thing I don't like is a rubber o-ring which limits the
number of gases which could be stored. I think for some application it should be replaced with o-ring made of PTFE, but this should be bought
separately.
Update: I've checked the description and it seams the o-ring in my bottle is from FKM. But I would say it looks like ordinary rubber.
[Edited on 3-7-2023 by teodor]
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
A quick Google search learned me stainless steel is OK for dry SO2:
ThyLabs has a video using CO2 paintball canisters:
https://www.youtube.com/watch?v=Q70Q5PCxn0c&ab_channel=T...
|
|
|