Synthesis of Werners Hexol
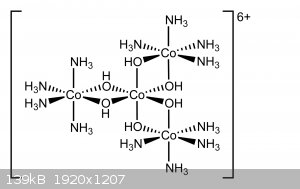
Following the synthesis procedure presented in "Inorganic chemistry" Volume 6 chapter 56 by Eugene G. Rochow (see post attachment), i was successfully
able to prepare Hexol sulfate. While not very interesting in terms of properties, the Hexol ion is of great significance historically, it was the
first chiral non-carbon compound to be discovered.
The procedure itself is split into 3 main parts, the first 2 of which are preparations of precursor coordination complexes.
Preparation of cis[Co(NH3)4(H2O)Cl]Cl2
2CoCl2•6H2O+2(NH4)2CO3 + 6NH3 + H2O2 -> 2[Co(NH3)4CO3]Cl + 2 NH3Cl + 14H2O
[Co(NH3)4CO3]Cl + 2HCl -> cis[Co(NH3)4(H2O)Cl]Cl2 + CO2
Into a 100 ml beaker was added 15 ml water and 16.2 ml 24% NH3. 4.1 g of NH4HCO3 was weighed in and the mixture stirred until dissolved. After
dissolution was complete, a solution of 2 g CoCl2•6H2O in 3 ml water was added with fast mechanical stirring. An initial precipitate forms, but this
quickly redissolves to leave a clear red solution.
Next, 5 ml of ~12% H2O2 was added dropwise with continued mechanical stirring over approximately 10 minutes. During the addition of peroxide, the
solution turns from red into a deep purple.

Stirring was continued for approximately 30 minutes until no more notable effervescence was seen. Next, the solution was transferred to a 250 ml
beaker and a thermocouple added, the hotplate was turned on (set to 120C) and a fan placed to blow over the solution to increase evaporation rate.
(The temperature of the solution should be kept at or below 80C)
Evaporation was continued until the volume of the solution was reduced to ~10 ml, this took about 1h 20 min. In order to prevent decomposition of the
complex, a solution of 0.6g NH4HCO3 and 0.5g 24% NH3 in 3 ml water was added 1 ml at a time every 30 minutes.

After evaporation, the hot solution was quickly filtered by vacuum, transferred to a 100 ml beaker and cooled in an ice bath. While keeping the
solution cool, 12.5 ml 6M HCl was added dropwise over a period of 5 minutes.

The solution was then removed from the ice bath and an additional 9 ml of 10M HCl added, which causes the carbonate complex to start precipitating. A
thermocouple was added to the solution and the solution heated to and kept at 60C for 1h. During the heating period, the color of the precipitate
changed from a red-ish to purple color as the carbonate decomposes and the chloride complex forms.

After heating, the suspension of crystals was transferred to a 100 ml Erlenmeyer flask, sealed by stopper and cooled by ice overnight. The next day,
the product crystals were collected by vacuum filtration, washed with 2x3ml Ethanol and dried in air.
Yield: 1.38g (65%)

Preparation of cis[Co(NH3)4(H2O)Cl]SO4
cis[Co(NH3)4(H2O)Cl]Cl2 + (NH4)2SO4 -> cis[Co(NH3)4(H2O)Cl]SO4 + 2NH4Cl
Into a 100 ml beaker was weighed 1.21g of 15% H2SO4 (0.1g pure sulfuric) and 30 g of water. 1 g of the previously prepared cis[Co(NH3)4(H2O)Cl]Cl2 was
added, and the mixture stirred for 30 min to dissolve as much as possible.

Undissolved chloride complex was removed by vacuum filtration.

The filtrate was transfered to a 100 ml beaker and 2.1g (NH4)2SO4 added. The mixture was stirred until all ammonium sulfate was dissolved, then placed
in an ice bath to crystallize for 1h.

Product crystals were collected by vacuum filtration, washed with 2x1ml ice cold water and 1 ml ethanol. Dried in air. Yield: 0.55g (96%)

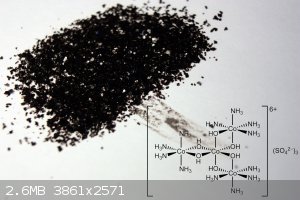
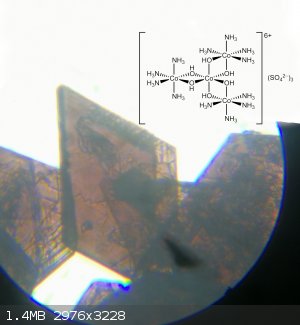
Preparation of Hexol Sulfate
4[Co(NH3)4(H2O)Cl]SO4 + 2NH3 + 6 H2O -> tris[Tetraamine-μ-dihydroxo-Cobalt(III)] Cobalt(III) Sulfate 4-hydrate + 4NH4Cl + (NH4)2SO4.
Into a 25 ml Erlenmeyer flask was added 15 ml water, 5g of ice and 0.3 ml 24% NH3. Once most of the ice had melted, 0.5g of the previously prepared
[Co(NH3)4(H2O)Cl]SO4 was added and the flask gently swirled to dissolve to dissolve the sulfate. Once dissolution was complete, the cold mixture was
set on a shelf in the shade at ambient temperature and left for 24h. During the 24h and as the solution heats up to room temperature, the hexol
sulfate product starts to crystallize and the solution darkens considerably
Note that its important that the mixture is not cooled after dissolution, as the crystallization and reaction occurs while the solution heats up.


The product crystals were isolated by vacuum filtration, washed with 3x1ml ice cold water and 1 ml ethanol. Dried in air. Yield: 0.28 g (65%)

Attachment: Hexol_Cobalt.pdf (1.2MB)
This file has been downloaded 199 times
|