Neal
Hazard to Others
  
Posts: 143
Registered: 24-12-2021
Location: Chicago, IL, USA.
Member Is Offline
|
|
Fluorescence/phosphorescence questions.
Fluorescence is supposed to be it emits light only when the light is shining to it, stops emitting as soon as the radiation is canceled.
Phosphorescence only emits the radiation as soon as the radiation is canceled.
So then, what do we call something that does both?
And if we use this as a Venn diagram, do most things that do 1 or the other, do both? Or are most compounds can only do 1?
What are some good examples of chemicals that can do phosphorescence and not fluorescence, or does fluorescence but not phosphorescence?
-
For a more complex question, supposedly phosphorescence is stronger at colder temperatures, but for fluorescence, there's cases it could go either
way?
I hear quantum dots cannot do much for phosphorescence, some of them can strongly fluoresce at room temperature but can only weakly phosphoresce at
colder temperatures?
|
|
|
Neal
Hazard to Others
  
Posts: 143
Registered: 24-12-2021
Location: Chicago, IL, USA.
Member Is Offline
|
|

And another question.
Here's a screenshot from Wikipedia, it was in 2017 when they accidentally discovered the 1st amphibian that has a fluorescence chemical for UV
light...
So do more things that fluoresce or phosphoresce, have not too wide of a radiation spectrum?
I imagine UV is the most common, IR is much less common? So if something fluoresces or phosphoresces under IR light, it's not likely going to also
fluorescence or phosphoresce under UV light? Unless it has a really wide spectrum for it?
Edit: what are the chances some chemical can only do fluorescence under UV light but also only do phosphorescence under IR light or so.
[Edited on 13-5-2023 by Neal]
|
|
|
JohnnyBuckminster
Harmless

Posts: 40
Registered: 6-6-2018
Member Is Offline
|
|
Quote: Originally posted by Neal  | Fluorescence is supposed to be it emits light only when the light is shining to it, stops emitting as soon as the radiation is canceled.
Phosphorescence only emits the radiation as soon as the radiation is canceled.
So then, what do we call something that does both?
|
Very simplified, I think you can see it like this: fluorescence and phosphorescence both happen simultaneously, i.e. phosphorescence does not start
when you turn off the light. Fluorescence and phosphorescence starts the same moment you turn ON the light.
The difference between fluorescence and phosphorescence is the time scale. Fluorescence typically occurs on a nanosecond time scale, so it might
appear instantaneous, but it is not. Phosphorescence, on the other hand, typically occurs on a microsecond time scale. So the moment you turn off the
light, fluorescence will emit for a nanosecond, and phosphorescence will shine on for microseconds, maybe even a second. It looks like it glows.
The probability for phosphorescence increases with decreasing temperature. At room temperature, most of the emission will be
fluorescence, but as the temperature is lowered, a larger fraction will come from phosphorescence. But it is important to realize, both process can
happen at room temperature! There is no clean cut.
Since both processes can occur simultaneously, and only differ in probability, it is not obvious how to draw a Venn diagram.
|
|
|
Neal
Hazard to Others
  
Posts: 143
Registered: 24-12-2021
Location: Chicago, IL, USA.
Member Is Offline
|
|
Found a contrary answer: There are plenty of chemicals that can both phosphoresce and fluoresce, or one or the other or neither. Its all about the
most efficient radiative and non-radiative decay pathways of the excited state molecule. When I freeze this one down to -77K in solution it does show
weak phosphoresence.
|
|
|
JohnnyBuckminster
Harmless

Posts: 40
Registered: 6-6-2018
Member Is Offline
|
|
Quote: Originally posted by Neal  | | Found a contrary answer: There are plenty of chemicals that can both phosphoresce and fluoresce, or one or the other or neither. Its all about the
most efficient radiative and non-radiative decay pathways of the excited state molecule. When I freeze this one down to -77K in solution it does show
weak phosphoresence. |
Not contradictory, but very simplified. There are multiple different pathways by which an excited molecule can deactivate back to the ground state.
It's all about the nature of the molecule, conditions, and probabilities.
|
|
|
Neal
Hazard to Others
  
Posts: 143
Registered: 24-12-2021
Location: Chicago, IL, USA.
Member Is Offline
|
|
Quote: Originally posted by JohnnyBuckminster  |
Not contradictory, but very simplified. There are multiple different pathways by which an excited molecule can deactivate back to the ground state.
It's all about the nature of the molecule, conditions, and probabilities. |
So, there's basically no pattern, to compounds that can fluoresce but not phosphoresce, or phosphoresce but not fluoresce?
[Edited on 19-5-2023 by Neal]
|
|
|
JohnnyBuckminster
Harmless

Posts: 40
Registered: 6-6-2018
Member Is Offline
|
|
Quote: Originally posted by Neal  | Quote: Originally posted by JohnnyBuckminster  |
Not contradictory, but very simplified. There are multiple different pathways by which an excited molecule can deactivate back to the ground state.
It's all about the nature of the molecule, conditions, and probabilities. |
So, there's basically no pattern, to compounds that can fluoresce but not phosphoresce, or phosphoresce but not fluoresce?
[Edited on 19-5-2023 by Neal] |
Photophysics is a complex topic. There are patterns in how a moleucle can deactivate from an excited state, often realted to steric hindrance the type
of groups present. Companies have been founded to explore different applications of moleucles undergoing radiateive decay. See for example Molecular
Probes, now owned by Fisher, who offers a wide range of probes for various applications, although the price is out of reach for most amateur
scientists. Take a look here: https://www.thermofisher.com/se/en/home/brands/molecular-probes.html"
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Neal  | ]
So, there's basically no pattern, to compounds that can fluoresce but not phosphoresce, or phosphoresce but not fluoresce?
[Edited on 19-5-2023 by Neal] |
There's a pattern, but it's complicated.
It gets worse when you include delayed fluorescence.
https://en.wikipedia.org/wiki/Thermally_activated_delayed_fl...
|
|
|
Neal
Hazard to Others
  
Posts: 143
Registered: 24-12-2021
Location: Chicago, IL, USA.
Member Is Offline
|
|
What about if we divided inorganic and organic compounds, those can do both or only 1, are the demographics any different? Are inorganic compounds
just as likely to do both or only 1, as organics?
|
|
|
JohnnyBuckminster
Harmless

Posts: 40
Registered: 6-6-2018
Member Is Offline
|
|
Quote: Originally posted by Neal  |
What about if we divided inorganic and organic compounds, those can do both or only 1, are the demographics any different? Are inorganic compounds
just as likely to do both or only 1, as organics? |
If you are speaking in front of an audience less familiar with the subject, you can get away by grouping molecules as fluorescent, phosphorescent, and
non-emitting. However, if someone in the audience starts asking more detailed questions like you are doing now, it will be challenging to continue
argue for such a strict grouping. You probably end up where we are now, it depends on the moleucle, on the surrounding polatiry, viscosity,
temperarure etc.
So, it depends on what you want to communicate. If you're aiming for a general overview, then yes, you can categorize molecules into three groups.
However, if you're going to examine things more closely, you're probably better off focusing on the different types of deactivation pathways.
|
|
|
Neal
Hazard to Others
  
Posts: 143
Registered: 24-12-2021
Location: Chicago, IL, USA.
Member Is Offline
|
|
Quote: Originally posted by JohnnyBuckminster  | Quote: Originally posted by Neal  |
What about if we divided inorganic and organic compounds, those can do both or only 1, are the demographics any different? Are inorganic compounds
just as likely to do both or only 1, as organics? |
If you are speaking in front of an audience less familiar with the subject, you can get away by grouping molecules as fluorescent, phosphorescent, and
non-emitting. However, if someone in the audience starts asking more detailed questions like you are doing now, it will be challenging to continue
argue for such a strict grouping. You probably end up where we are now, it depends on the moleucle, on the surrounding polatiry, viscosity,
temperarure etc.
|
I would make this for room-temperature only.
Also.
For phosphorescence, an electron can flip its spin, where you have, say, 2 “up” electrons, and you can have “triple” the amount of
arrangements. You can have both up, both down, or both kinda sideways. This is the triplet state. Since the spins are no longer paired, it’s hard
for the molecule to relax, and it stays excited for longer while it waits for its spins to pair back up.
But for fluorescence, it's the same as molecules don't emit either, they don't emit photons when they relax. All their electrons are paired together
so their up and down spins cancel out, and that's fluorescence.
Is there any way to categorize the 3?
I would also not include complex organic molecules. If you take a large organic molecule that primarily phosphoresces, and a large organic molecule
that primarily fluoresces, and then combined them with some alkyl chain, then the new molecule would do both. So I'm more fascinated with inorganic,
ionic, and small/simpler organic compounds.
[Edited on 21-5-2023 by Neal]
[Edited on 21-5-2023 by Neal]
|
|
|
Keras
National Hazard
   
Posts: 895
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
To me, phosphorescence is linked to a chemical reaction. P emits light because of spontaneous oxidation to P₂O₅ in oxygen. That’s where the
energy stems from.
Fluorescence is, as the others explained, photoemission linked to the return of a molecule to its ground state after it’s been excited by a
high-energy (low wavelength) burst of light. In other words, a fluorescent product won’t shine spontaneously in the dark, but a phosphorescent one
will.
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
That's chemiluminescence.
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Neal  |
I would also not include complex organic molecules. If you take a large organic molecule that primarily phosphoresces, and a large organic molecule
that primarily fluoresces, and then combined them with some alkyl chain, then the new molecule would do both.
[Edited on 21-5-2023 by Neal] |
Not necessarily, you can get energy coupling through space where the part of the molecule that emits light s not the same as the part that absorbs it.
|
|
|
Keras
National Hazard
   
Posts: 895
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Alright. So phosphorus is not phosphorescent :p
|
|
|
Neal
Hazard to Others
  
Posts: 143
Registered: 24-12-2021
Location: Chicago, IL, USA.
Member Is Offline
|
|
Quote: Originally posted by unionised  | Quote: Originally posted by Neal  |
I would also not include complex organic molecules. If you take a large organic molecule that primarily phosphoresces, and a large organic molecule
that primarily fluoresces, and then combined them with some alkyl chain, then the new molecule would do both.
[Edited on 21-5-2023 by Neal] |
Not necessarily, you can get energy coupling through space where the part of the molecule that emits light s not the same as the part that absorbs it.
|
What do we call this? And can you think of a relatively small molecule that does it?
|
|
|
Neal
Hazard to Others
  
Posts: 143
Registered: 24-12-2021
Location: Chicago, IL, USA.
Member Is Offline
|
|
Quote: Originally posted by unionised  | Quote: Originally posted by Neal  |
I would also not include complex organic molecules. If you take a large organic molecule that primarily phosphoresces, and a large organic molecule
that primarily fluoresces, and then combined them with some alkyl chain, then the new molecule would do both.
[Edited on 21-5-2023 by Neal] |
Not necessarily, you can get energy coupling through space where the part of the molecule that emits light s not the same as the part that absorbs it.
|
Does fluorescence and phosphorescence require the same electrons to emit, must be the same electrons to absorb?
|
|
|
Neal
Hazard to Others
  
Posts: 143
Registered: 24-12-2021
Location: Chicago, IL, USA.
Member Is Offline
|
|
You know what scratch some of the above questions, I didn't realize glow-in-the-dark toys are not fluorescence/phosphorescence. They are persistent
luminescence, which is also different than chemiluminescence.
I probably don't care as much who can fluorescence and phosphoresce at the same time, or who does 1 or the other.
I'm not too interested in UV light either. My next set of questions are.
Who fluoresces and phosphoresces under IR light? And can any that fluoresce and phosphoresce under IR light, also fluoresce/phosphoresce under UV
light?
|
|
|
Neal
Hazard to Others
  
Posts: 143
Registered: 24-12-2021
Location: Chicago, IL, USA.
Member Is Offline
|
|
You know, I finally just asked at the Chemistry Stack Exchange. And someone said there is no pattern. Weird, I been banned from the Chemistry Stack
Exchange for about 2 years, but the Chemistry Stack Exchange didn't unban me, their parent company did, after appealing to them all this time.
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by Neal  | | Who fluoresces and phosphoresces under IR light? And can any that fluoresce and phosphoresce under IR light, also fluoresce/phosphoresce under UV
light? |
Are you asking what materials can absorb infrared light and emit visible light? I'm not sure anything is able to do that, at least not in the way that
UV fluorescence/phosphorescence works.
That behavior might be described by 'candoluminescence', however.
|
|
|
Neal
Hazard to Others
  
Posts: 143
Registered: 24-12-2021
Location: Chicago, IL, USA.
Member Is Offline
|
|
Quote: Originally posted by SnailsAttack  | Quote: Originally posted by Neal  | | Who fluoresces and phosphoresces under IR light? And can any that fluoresce and phosphoresce under IR light, also fluoresce/phosphoresce under UV
light? |
Are you asking what materials can absorb infrared light and emit visible light? I'm not sure anything is able to do that, at least not in the way that
UV fluorescence/phosphorescence works.
That behavior might be described by 'candoluminescence', however. |
No, that would be anti-Stokes.
I'd be curious for something that absorbs deep-IR and emits microwaves.
As far as absorb near-IR and emit deep-IR, I realize that is redundant now, because almost everything at room temperature emits IR, not from
fluorescence/phosphorescence, but from being a blackbody.
But I am interested in absorb and emit in the visible spectrum, i.e., absorb violet and emit red.
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
It's pretty simple if you draw it out a Jabłonski diagram.
Fluorescence is when a singlet excited state decays to the ground state and emits a photon.
Phosphorescence is when the singlet excited state undergoes intersystem crossing to a triplet state, which then decays to the (singlet) ground state
and emits a photon. Since this is actually a "forbidden" transition, it's much slower, hence the delay in emission.
See: https://chem.libretexts.org/Bookshelves/Physical_and_Theoret...
[Edited on 2023-7-30 by Metacelsus]
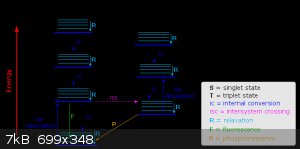
|
|
|
Neal
Hazard to Others
  
Posts: 143
Registered: 24-12-2021
Location: Chicago, IL, USA.
Member Is Offline
|
|
Quote: Originally posted by Metacelsus  | It's pretty simple if you draw it out a Jabłonski diagram.
Fluorescence is when a singlet excited state decays to the ground state and emits a photon.
Phosphorescence is when the singlet excited state undergoes intersystem crossing to a triplet state, which then decays to the (singlet) ground state
and emits a photon. Since this is actually a "forbidden" transition, it's much slower, hence the delay in emission.
See: https://chem.libretexts.org/Bookshelves/Physical_and_Theoret...
[Edited on 2023-7-30 by Metacelsus] |
The website says, "Fluorescence rarely results from absorption of ultraviolet radiation of wavelength shorter than 250 nm because radiation at this
wavelength has sufficient energy to deactivate the electron in the excited state by predissociation or dissociation."
I'd like to know what the other end limit is, how long can the wavelength be for fluorescence, and more importantly phosphorescence happen?
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
I've used fluorophores excited at 756nm (Cy7). If you take two-photon absorption into account, you could double this to 1512 nm, although your light
intensity would need to be much stronger.
For fluorescence, the photon needs to have enough energy to excite an electronic state. So the question becomes, what's the lowest-energy electronic
excitation?
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Check out https://www.fpbase.org/spectra/ and you can see lots of different fluorophores (mostly ones used for biological research).
|
|
|