SplendidAcylation
Hazard to Others
  
Posts: 203
Registered: 28-10-2018
Location: Starving in some deep mystery
Member Is Offline
Mood: No one I think is in my tree.
|
|
Preparation of isobutyrophenone by the addition of isopropyl Grignard reagent to benzonitrile (Photo-assay)
Hi,
As promised, I have finally completed my write-up for this reaction!
The write-up below is copied directly from the PDF version, which will be added as an attachment shortly.
I am unable to attach the PDF version with pictures, due to the file size, so I will attach the text-only version instead.
A few things worth mentioning before the start of the write-up:
You will notice a lack of pictures in the Work-up section; This is because I forgot to take any!
I was so preoccupied with doing the actual procedure, that I completely forgot to take any pictures; I hope my powers of description will fill in the
gaps and allow the construction of a mental picture! 
Also a small point relating to the smell of isobutyrophenone.
Despite the "butyro" part, it is not an unpleasant smell at all, in my opinion.
I have never had the joy of smelling any other aromatic ketones, so I don't know whether it is similar or not to, for instance, acetophenone.
Isobutyrophenone has a very "heavy", somewhat sweet, somewhat floral, smell...
It is not a "fresh" floral smell like phenethyl alcohol, nor is it a fruity ester-like smell.
It is not really similar to the smell of benzaldehyde or benzonitrile, in the sense that it doesn't smell like almonds, however it is
somewhat similar in the sense that it is quite a heavy, aromatic smell.
Anyway, I hope this is useful to someone, it certainly took a lot of work to get all the pictures resized and put in the right place!
I have not found much discussion of the preparation of ketones by the Grignard reaction on ScienceMadness, so I hope this will help anyone considering
a similar reaction.
And, of course, please let me know if I've made any mistakes 
Preparation of isobutyrophenone by the addition of isopropyl Grignard to benzonitrile
Introduction:
There are numerous preparative routes to aromatic ketones; For example, the Friedel-Crafts acylation reaction, the dry-distillation of a mixture of
benzoic acid, the corresponding carboxylic acid, and iron[1][2], among many other routes.
However, the addition of a Grignard reagent to benzonitrile is a less well-known route.
The prototypical Grignard reaction involves the addition of a Grignard reagent to a carbonyl carbon, although Grignard reagents will also add to a
nitrile carbon, yielding a deprotonated imine.
This deprotonated imine can then be hydrolysed to the imine, to the iminium ion, and finally to a ketone; This is the procedure carried out here.
The Grignard approach has the advantages of being very versatile and the procedure can probably be adapted to prepare any alkyl phenyl ketone,
providing the corresponding alkyl halide is available.
Moreover, it doesn’t require hard-to-obtain acyl halides, unlike the Friedel-Crafts acylation reaction.
An illustrative example of a Grignard reagent being added to benzonitrile is the preparation of diphenyl ketimine in Organic Syntheses[3], in this
procedure, the hydrolysis is stopped at the imine, rather than carrying out full hydrolysis to the ketone.
Preparation of reagents:
The solvents and reagents used below were all dried over molecular sieves prior to the preparation of these solutions, whereupon further molecular
sieves were added, as indicated below.
A solution of (7.15g, 58.1mmol) isopropyl bromide in 20mL ether was prepared in a brown-glass bottle, to which 3g molecular sieves were added.
A solution of 4.80g (46.5mmol, 80% theoretical) benzonitrile in 7mL ether was prepared, to which 0.8g molecular sieves were added.
These solutions were transferred to a sealed plastic bag and stored in the fridge until use.
1.5g of magnesium ribbon (61.7mmol) was sanded with emery paper to remove corrosion, cleaned with isopropanol, and cut into ~5mm2 pieces.
A 3-neck 500mL RBF, Graham condenser, stopper, thermometer adapter (to convey the pipette), three pipettes, a bung through which a glass tube was
inserted*, a small bung, a stir-bar, and the magnesium pieces, were dried in the oven at gas-mark 1 for 45 minutes.
Upon removal from the oven, the potentially moist air was sucked out of the apparatus using a vacuum-cleaner.
The reaction steps are given below:
Formation of Grignard reagent:
(CH3)2CHBr + Mg → (CH3)2CH-Mg-Br
-----------------...------....-----------------------
....122.99........24.31..........147.30
Nucleophilic addition of Grignard reagent to benzonitrile, forming the deprotonated imine:
(CH3)2CH-Mg-Br + C6H5CN ----> ((CH3)2CH)(C6H5)C=N- + Mg2+ + Br-
------------------------...------------
........147.30..............103.12
Protonation of the deprotonated imine, forming the imine:
((CH3)2CH)(C6H5)C=N- + HX ----> ((CH3)2CH)(C6H5)C=NH + X-
Protonation of the imine:
((CH3)2CH)(C6H5)C=NH + HX ----> ((CH3)2CH)(C6H5)C=NH2+ + X-
Hydrolysis of the imine to the end-product, the ketone, isobutyrophenone:
((CH3)2CH)(C6H5)C=NH2+ + H2O ----> ((CH3)2CH)(C6H5)C=O + NH4+
.................................................................--------------------------------
................................................................................148.20
Preparation of the Grignard reagent, and its addition to benzonitrile:
Figure 1 (left): The apparatus
Figure 2 (right): Magnesium ribbon and stir-bar in flask
 
The apparatus was set up as follows:
The RBF, with the stir-bar therein, was placed on the hot-plate in an Al-foil air-bath, the Graham condenser was attached to the centre neck, along
with the thermometer adapter and stopper in the side-necks.
The joints were greased with Vaseline.
A drying-tube filled with CaCl2 granules was attached to the top of the condenser using the bung (marked above with *).
The thermometer adapter was stoppered using the small bung.
The magnesium pieces, along with a small granule of iodine, were placed in the flask, and the flask was heated with a heat-gun to vapourise the
iodine, coating the magnesium, in order to activate the magnesium to enable it to react with the alkyl halide more readily.
Figure 3: After vapourisation of the iodine

Magnetic stirring was commenced, whereupon the ethereal isopropyl bromide was then added, portion-wise, via a pipette inserted through the thermometer
adapter, followed by expedient replacement of the rubber-bung.
The entirety of the solution was added over 30 minutes.
Figure 4 (left): First signs of the formation of the Grignard reagent (bubbling)
Figure 5 (right): Grignard reagent continuing to form
 
Figure 6: Grignard reagent fully formed, with unreacted magnesium visible

The solution was then heated intermittently while stirring for 30 minutes.
The ethereal benzonitrile solution was then added over a 15 minute period.
Figure 7 (left): After first benzonitrile addition
Figure 8 (right): The reaction mixture after all of the benzonitrile had been added
 
The flask was then heated intermittently, with stirring, for one hour.
The mixture was then left to stand for around three hours, at room-temperature, until time was available to carry out the refluxing.
The mixture was then refluxed on a water-bath at 50°C
Figure 9: Refluxing the reaction mixture
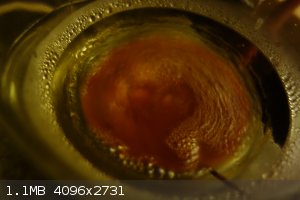
For 30 minutes, no visible change was observed, however, suddenly, after around 30 minutes of refluxing, a large quantity of precipitate had formed.
Figure 10: Stirring temporarily discontinued, showing the grey-brown precipitate settled to the bottom of the flask

Refluxing was continued for another three-and-a-half hours, making a total of four hours, whereupon heating was discontinued, and the mixture was left
at room-temperature overnight.
Hydrolysis of the addition compound:
By the next day, the precipitate had settled into a red/brown layer at the bottom.
Figure 11: The precipitated iminium salt, still anhydrous, having settled overnight

A few millilitres of water were added, whereupon a strong exotherm was observed, with the formation of white magnesium hydroxide.
Figure 12: Water added to the flask, white magnesium hydroxide forming due to hydrolysis

It was not necessary to add a stoichiometric quantity of water to hydrolyse the imine salt, as this would be done when acid was added subsequently.
A solution of 18g of 35% HCl in 20mL water (174mmol, a 50% excess) was prepared and cooled in the freezer.
The acid was then added slowly, via one of the side-necks.
With each addition, a considerable exotherm took place; Since no ice was available to cool the condenser, and since the acid was being added via a
side-neck, this had to be done in small quantities, with the stopper being replaced expediently after each addition.
Figure 13: The reaction mixture after some acid had been added

Addition of the acid was accompanied by considerable effervescence, due to boiling ether and hydrogen produced by the reaction of Mg with the acid.
Once all of the acid had been added, a biphasic solution was obtained, with a small piece of unknown material remaining unreacted.
The lower (aqueous) phase was strongly acidic, so it was unlikely that the unknown material was unreacted magnesium.
Figure 14: Reaction mixture now biphasic, after all of the acid had been added.

Refluxing to complete hydrolysis:
The mixture was left to stand overnight, whereupon it was refluxed, with strong stirring on a water bath at 40°C, for 20 minutes.
At this point, the small piece of unknown material was still undissolved, so ~2mL 35% HCl was added and refluxing was continued for 40 minutes.
The material still didn’t dissolve, however.
Work-up:
A 5% solution (2g in 40mL water) of sodium bicarbonate was prepared and cooled in the freezer.
The post-reflux biphasic mixture was transferred to a separatory funnel and the lower aqueous layer was drained off.
The ether layer was transferred to a beaker, and the aqueous phase was returned to the funnel and washed with 5x5mL ether, the ether extracts being
combined with the original ether phase in the beaker.
The ethereal solution was then washed with 2x20mL 5% sodium bicarbonate solution (slight effervescence was observed during the first wash).
The ethereal solution was then washed with 20mL cold water.
At this point, it was remembered that 0.97g crude isobutyrophenone from a previous reaction had been retained, so this was added to the solution at
this stage, in order that it might be purified alongside the newly produced ketone.
The crude isobutyrophenone added probably contained traces of acetonitrile, water, and amines.
A final cold-water wash was carried out to remove these potential impurities (in retrospect it would have been better to add the crude
isobutyrophenone to the ethereal solution at the beginning of the work-up).
The ethereal solution was then transferred to a volumetric flask alongside 2g anhydrous MgSO4 and 0.5g CaCO3 (to neutralize any remaining traces of
acid) and stored in the fridge.
Suction-filtration of ethereal isobutyrophenone:
The next day, the dried ethereal solution was suction-filtered, via a Gooch crucible, into a 500mL 3-neck RBF.
The volumetric flask was rinsed out twice with ether, this was also filtered via the Gooch crucible.
Figure 15: The dried, ethereal solution of isobutyropheonone, prior to distillation

Distillation of the ethereal isobutyrophenone:
The apparatus was then arranged for simple distillation using a short-path distillation apparatus with ice-water pumped through the condenser, and a
stir-bar in the distilling-flask.
Figure 16: The distillation apparatus

An air-bath using Al-foil was used, with the PID set to 100°C, and magnetic stirring was commenced.
Most of the ether was thus distilled off.
Once the distillation rate slackened, the hot-plate was switched to full-power, and the stopper in the still-head was replaced by a thermometer
adapter carrying a thermocouple, connected to the PID controller, to display the temperature.
Everything up to 42°C was collected and considered to be reasonably pure ether; this was decanted, whereupon the receiving flask was reattached.
The temperature then slowly rose to about 50°C with a few drops coming over, and then rapidly to around 180°C, whereupon the receiver was swapped
and isobutyrophenone began to distil over at 200-202°C.
The intermediate fraction was suspected to be the aforementioned traces of acetonitrile.
Figure 17 (left): Isobutyrophenone vapour rising in still-head
Figure 18 (right): The still-head temperature as the isobutyrophenone distils over
 
After a few minutes distilling at 200-202°C, the distillation rate slackened somewhat, and the liquid that came over thereafter was brown-amber.
Figure 19: The amber distillate

After a short period, no significant quantities of ketone remained in the distilling flask, with a dark, oily, viscous residue remaining.
The distillation was thus complete, with a second distillation being necessary to purify the amber product.
Yield of crude product: 4.6g
Figure 20: The tarry residue in the distilling flask

Re-distillation of the isobutyrophenone:
The apparatus was arranged with a short-path distillation head, 250mL distilling flask, and 150mL receiving flask.
Some anti-bumping granules were placed in the distilling flask and the slightly yellow ketone was transferred to the flask.
Figure 21: The yellow isobutyrophenone in the distilling flask, prior to the second distillation

Tap-water was pumped through the condenser, and the hot-plate was switched on high-power.
An air-bath, using Al-foil, was used.
After about 10 minutes, the granules were heard rattling inside the flask, indicating the substance was refluxing.
The pump was switched off in order to prevent the apparatus from cooling too much, thus encouraging the vapour to come over (there was probably no
need for a continuous flow of cold water in the condenser, as the ketone has a high boiling-point)
After around 30 minutes, the ketone began to come over as a clear liquid, at 207°C.
Figure 22 (left): A drop of the clear distillate
Figure 23 (right): Isobutyrophenone distilling over at 207°C
 
Figure 24: Tarry residue in distilling flask

The final yield of isobutyrophenone was 3.10g.
Subtracting the added 0.97g, this corresponds to a yield, of this reaction, of 2.13g (14.37mmol), a percentage yield of 30.9%.
The isobutyrophenone was transferred to a brown-glass bottle containing previously prepared isobutyrophenone, alongside some MgSO4 desiccant.
Conclusions:
The action of Grignard reagents upon benzonitrile is an effective preparative route to phenyl ketones, the preparation of isobutyrophenone given here
being a representative example of this reaction.
This reaction has been carried out twice before under similar conditions, the first time using 39mmol of isopropyl magnesium bromide, and the second
time using 89mmol of isopropyl magnesium chloride; This write-up representing the third attempt at this reaction, the first two being documented
elsewhere.
The percentage yield of the first two attempts combined was 48.5%, so the 30.9% yield obtained here was somewhat low, however the yield may have been
underestimated, as the crude isobutyrophenone added probably accounted for less than 0.97g, due to impurities, and therefore the yield of the reaction
was probably correspondingly higher.
The apparatus used here was not ideal; In the first attempt at this reaction, a Liebig condenser was used, and the reactants were added down the
condenser.
This worked well, but the condenser was not sufficient to condense the refluxing ether without losses, so, in the second attempt at this reaction, an
addition funnel was used to add the reactants to the flask, and a Graham condenser was used to condense the ether (this type of condenser is not
designed for reflux, however it did work remarkably well).
This approach was better, however the only addition funnel available was a 1000mL one, much too large for the small quantities of solutions used.
Due to these problems, the apparatus used in this write-up was devised, the idea was that the Graham condenser would be used to condense the refluxing
ether, while the reactants would be added, via a pipette, through the thermometer adapter.
However, a major problem that had been overlooked was that significant quantities of ether vapour would escape via the thermometer adapter during
additions of reactants.
In the future, it would be better to use a more appropriate condenser, alongside a smaller addition funnel.
References:
[1] “Thermal Decarboxylation - Alkyl Phenyl Ketones” (http://www.sciencemadness.org/talk/viewthread.php?tid=3459#p...)
[2] “Decarboxylation Studies. II. Preparation of Alkyl Phenyl Ketones” - Charles Granito and Harry P. Schultz
(https://pubs.acs.org/doi/abs/10.1021/jo01038a521)
[3] “diphenyl ketimine” (https://www.orgsyn.org/demo.aspx?prep=cv5p0520)
[Edited on 31-3-2023 by SplendidAcylation]
[Edited on 31-3-2023 by SplendidAcylation]
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Thanks for the nice write-up! For addition of the reagents to the flask, I recommend you buy some rubber septum stoppers and disposable
syringes/needles. These are fantastic for adding solutions to your reaction without opening the apparatus. Reusable glass syringes are also an option,
but the cheaper ones are terrible and not worth it.
|
|
|
Texium
|
Thread Moved
31-3-2023 at 07:49 |
SplendidAcylation
Hazard to Others
  
Posts: 203
Registered: 28-10-2018
Location: Starving in some deep mystery
Member Is Offline
Mood: No one I think is in my tree.
|
|
PDF version
Thanks Tex!
That's a good idea; Do you suppose plastic syringes will be able to handle ether?
I thought perhaps the rubber seal might expand and make the plunger almost impossible to move in or out, indeed, that does happen with some solvents.
My rationale behind not using the 1000mL addition funnel is as follows;
1) Even if I oven dry the funnel, the glass is bound to retain some water, and since the surface area of the glass is so high, it is bound to
get into the solution inside the funnel.
2) The large space in the funnel will fill with ether and halide vapour, resulting in loss of the liquids.
3) The air in the funnel is likely not to be completely dry, and water will diffuse from the air into the solution inside the funnel.
That said, I did use it before, and it seemed to work fine; Do you suppose my worries are unfounded?
P.S. I have attached the PDF, text-only version, in case this is more convenient for anyone.
P.P.S. I have been meaning to ask this for some time but I keep forgetting;
What are the rules that determine whether I might edit a post or not?
I see I am no longer able to edit my original post (which is fine, ironically the only reason I was going to edit it was to remove the extraneous
"[Edited by...]" tag at the bottom).
Is it impossible to edit any post once a reply has been posted?
Attachment: Grignard no images for SM.pdf (52kB)
This file has been downloaded 200 times
[Edited on 2-4-2023 by SplendidAcylation]
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
I wouldn’t recommend plastic syringes with rubber seals. The ones I use are all HDPE with no rubber. They still seal incredibly well and are
resistant to virtually all solvents. I’ve even used them with DCM, chloroform, DCE without issue. I wouldn’t reuse them after using them with a
halogenated solvent.
Now that I look for them though, I see that they are incredibly hard to find from companies that sell to individuals. Sometimes I take things for
granted working in a university research lab.
These are the syringes in question: https://www.henkesasswolf.de/en/veterinary/products/single-u...
I found a couple reasonably priced boxes on eBay.
10 mL: https://www.ebay.com/itm/115440111038?_trkparms=amclksrc%3DI...
1 mL: https://www.ebay.com/itm/144302111627?_trkparms=amclksrc%3DI...
Regarding the addition funnel, I think your concerns are warranted. I wouldn’t use a funnel that large on this scale of reaction. In fact, I’d
recommend using a significantly smaller flask for the reaction if you can. My rule of thumb is 2-4x the volume of solvent I’m using. You used 27 mL
of solvent in a 500 mL flask. I’d have probably used a 50 or 100 mL flask. I understand if you don’t own 3-necked flasks of that size, but if
that’s the case, a couple of those and a 14/20 condenser would be a worthy investment if you plan to do more small-ish scale organic synthesis.
As for editing posts, unless you’re a mod or admin, you can’t edit posts after 24 hours. That rule is in place to prevent people from “changing
history” or annoyingly deleting their threads after people have replied.
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
SplendidAcylation - very nice experiment !!! Thanks for sharing. Reaction of Grignard reagent with nitriles is not so well known as with carbonyl
compounds and organic halides. Moreover preparing a ketone product seems to possible only from a nitrile like you did. Reacting a Grignard reagent
with an ester / acid halide / acid anhydride would produce a ketone but the ketone would undergo further reaction to alcohol.
Sorry for 2 off topic questions:
1. Recently I've bought 14/23 and 29/32 silicone septa, so did I well buying them because there were also cheaper rubber septa. Moreover the 14/23 are
already sold out and only 29/32 available (or rubber material is in stock too). These silicone septa are Glassco, I see that brand on both paper boxes
(the Chemland noted at the internet page seems to be a mistake).
https://sklep-chemland.pl/en/septa-do-szlifow-z-silikonu-nr-...
2. In my country, there are plenty of various brand of medicinal syringes from polypropylene and polyethylene, like this:
https://www.asker.cz/injekcni-strikacky-jehly/strikacka-inje...
But not HDPE, seems the HDPE material is used only in chemistry (in medicine we do not use organic solvents so that's why not HDPE) ??? For which
solvents these polypropylene and polyethylene could be OK to use?
|
|
|
SplendidAcylation
Hazard to Others
  
Posts: 203
Registered: 28-10-2018
Location: Starving in some deep mystery
Member Is Offline
Mood: No one I think is in my tree.
|
|
Quote: Originally posted by Texium  | I wouldn’t recommend plastic syringes with rubber seals. The ones I use are all HDPE with no rubber. They still seal incredibly well and are
resistant to virtually all solvents. I’ve even used them with DCM, chloroform, DCE without issue. I wouldn’t reuse them after using them with a
halogenated solvent.
Now that I look for them though, I see that they are incredibly hard to find from companies that sell to individuals. Sometimes I take things for
granted working in a university research lab.
These are the syringes in question: https://www.henkesasswolf.de/en/veterinary/products/single-u...
I found a couple reasonably priced boxes on eBay.
10 mL: https://www.ebay.com/itm/115440111038?_trkparms=amclksrc%3DI...
1 mL: https://www.ebay.com/itm/144302111627?_trkparms=amclksrc%3DI...
Regarding the addition funnel, I think your concerns are warranted. I wouldn’t use a funnel that large on this scale of reaction. In fact, I’d
recommend using a significantly smaller flask for the reaction if you can. My rule of thumb is 2-4x the volume of solvent I’m using. You used 27 mL
of solvent in a 500 mL flask. I’d have probably used a 50 or 100 mL flask. I understand if you don’t own 3-necked flasks of that size, but if
that’s the case, a couple of those and a 14/20 condenser would be a worthy investment if you plan to do more small-ish scale organic synthesis.
As for editing posts, unless you’re a mod or admin, you can’t edit posts after 24 hours. That rule is in place to prevent people from “changing
history” or annoyingly deleting their threads after people have replied. |
Ooh syringes without rubber, interesting!
Why wouldn't you re-use them after transferring halogenated solvents?
Yes indeed, I think some smaller flasks would be useful.
The editing rule makes sense, I'll keep that in mind in the future 
Quote: Originally posted by Fery  | SplendidAcylation - very nice experiment !!! Thanks for sharing. Reaction of Grignard reagent with nitriles is not so well known as with carbonyl
compounds and organic halides. Moreover preparing a ketone product seems to possible only from a nitrile like you did. Reacting a Grignard reagent
with an ester / acid halide / acid anhydride would produce a ketone but the ketone would undergo further reaction to alcohol.
Sorry for 2 off topic questions:
1. Recently I've bought 14/23 and 29/32 silicone septa, so did I well buying them because there were also cheaper rubber septa. Moreover the 14/23 are
already sold out and only 29/32 available (or rubber material is in stock too). These silicone septa are Glassco, I see that brand on both paper boxes
(the Chemland noted at the internet page seems to be a mistake).
https://sklep-chemland.pl/en/septa-do-szlifow-z-silikonu-nr-...
2. In my country, there are plenty of various brand of medicinal syringes from polypropylene and polyethylene, like this:
https://www.asker.cz/injekcni-strikacky-jehly/strikacka-inje...
But not HDPE, seems the HDPE material is used only in chemistry (in medicine we do not use organic solvents so that's why not HDPE) ??? For which
solvents these polypropylene and polyethylene could be OK to use? |
Thanks!
Yes, in this reaction you end up with an imine, which of course will hydrolyse to the ketone.
Another approach is to prepare the secondary alcohol, and then oxidise it to the ketone, of course, but that isn't so convenient.
Moreover, if I were to prepare the secondary alcohol I'd have to use benzaldehyde to add the Grignard reagent to, and I've no idea whether it would be
easy to prepare pure benzaldehyde free from benzoic acid for this.
As for the syringes, polyethylene is the PE part in HDPE, so maybe polyethylene is the same as HDPE?
Furthermore, polypropylene is just polyethylene with a methyl group on every second carbon in the chain, I can't imagine it being drastically
different in terms of chemical resistance, but I really don't know! 
You can find chemical resistance charts like this one:
https://www.plasticsintl.com/chemical-resistance-chart
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Your way of preparing ketone is very nice.
Benzaldehyde is very cheap
https://sklep-chemland.pl/en/odczynniki-chemiczne/odczynniki...
https://shop.es-drei.de/aldehyde/11571/benzaldehyd-min.-99-5...
The property of polymer depends on molecule size and additives. These medicinal syringes are designed for diluted water solutions. I wonder whether
someone used them successfully for some organic solvents.
|
|
|
|