| Pages:
1
2 |
Gammatron
Hazard to Others
  
Posts: 125
Registered: 30-8-2022
Location: Abandoned Uranium Mines
Member Is Offline
|
|
Mystery oxidizer labeled as KNO3
About a year ago I purchased a bunch of "KNO3" on eBay. Yesterday I finally got around to trying to distill some HNO3 with it and got nothing but a
bunch of foam and dense white fumes, no reaction... I posted about it here thinking something went wrong with my reaction but it turns out my KNO3 isnt KNO3 at all. It is definitely an oxidizer but can't figure out
which so I will just refer to it as Ox.
Here is what I've found out about it:
- It is a fine clumpy white powder, much like KNO3
- It burns hot, bright and fast with sugar in 2:1 - Ox:sugar, very similar to KNO3.
- It does not react with H2SO4, NaOH or NaOCl
- Is somewhat hydrophobic and will not dissolve in water until it is near boiling, recrystallizes as very fine dense particles.
- It melts and decomposes into white smoke over a propane flame but a lighter flame isn't hot enough to melt it. Little to no residue left behind
I made flash powder with it 5:3:2 - Ox:Al:S
- It cannot be ignited with heat as it decomposes
- It is somewhat sensitive to shock and a good blow from a hammer makes a bright and very loud blast, even with just a pinch
I am rather impressed with its pyrotechnical properties but I am completely dumbfounded as to what it might be. Any help is appreciated and big thanks
to everyone that helped me thus far on my other post!
[Edited on 3-23-2023 by Gammatron]
|
|
|
Rainwater
National Hazard
   
Posts: 936
Registered: 22-12-2021
Member Is Offline
Mood: Break'n glass & kick'n a's
|
|
Is it soluble in ethanol?
"You can't do that" - challenge accepted
|
|
|
Gammatron
Hazard to Others
  
Posts: 125
Registered: 30-8-2022
Location: Abandoned Uranium Mines
Member Is Offline
|
|
Not soluble in ethanol or acetone altho it does stay in suspension longer because the solvent is able to get into the clumps of fine powder which
water cannot.
|
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
No rxn with sulfuric acid makes me rule out chlorate.
Poor solubility in water makes me lean away from most of the nitrates.
What do you mean that flash with it does not ignite with heat? I know you said it decomposes, but how?
Also, when you did the sugar test did you get a hint of color to point to the cation?
The water solubility makes me think it's potassium perchlorate. At least that would be me first guess from all of this.
|
|
|
Parakeet
Hazard to Self
 
Posts: 75
Registered: 22-12-2022
Location: Japan
Member Is Offline
Mood: V (V)
|
|
Maybe it’s potassium persulfate.
It is sparingly solubility in water, decomposition, insoluble in ethanol.
|
|
|
Rainwater
National Hazard
   
Posts: 936
Registered: 22-12-2021
Member Is Offline
Mood: Break'n glass & kick'n a's
|
|
https://en.m.wikipedia.org/wiki/Category:Pyrotechnic_oxidize...
After reading this list.
White salts with low solubility include
Barium peroxide
Strontium peroxide
This list is questionable. It list ptfe as an oxidizer? Subject for another topic
"You can't do that" - challenge accepted
|
|
|
Gammatron
Hazard to Others
  
Posts: 125
Registered: 30-8-2022
Location: Abandoned Uranium Mines
Member Is Offline
|
|
Did a bigger burn test with sugar than I did before and the flame was white hot and it and the smoke was slightly purple so for a while I was sure it
was KNO3 or KClO4. I do have KClO4 tho and I compared it with the unknown powder and there were noticeable differences in the burn rate and some
reaction tests I did. When I tried to ignite the flash with a flame it basically just melted and smoked leaving a residue of the Al powder.
Wikipedia: Potassium persulfate is the inorganic compound with the formula K2S2O8. Also known as potassium peroxydisulfate, it is a white solid that
is sparingly soluble in cold water, but dissolves better in warm water. This salt is a powerful oxidant
This actually seems like a very likely candidate, also because it is a sulfate it would not react with H2SO4. Everything lines up with this so far,
does anyone know any specific tests I could do to confirm this?
Edit: the scimad wiki states that HNO3 decomposes it to oxygen and nitrogen but I didnt notice any reaction with it even with heating, altho I
wouldn't say that rules it out.
[Edited on 3-23-2023 by Gammatron]
|
|
|
Rainwater
National Hazard
   
Posts: 936
Registered: 22-12-2021
Member Is Offline
Mood: Break'n glass & kick'n a's
|
|
https://www.peroxychem.com/media/22874/fmc_peroxygen_talk_20...
Wiki says it decomposes releasing O2 above 100c. You can measure the mass of a sample and heat it. Perform a splint test for O2, then measure the mass
afterwards.
[Edited on 23-3-2023 by Rainwater]
"You can't do that" - challenge accepted
|
|
|
ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
There is a way to determine if it's K2S2O8. Make a wet slush of it and MnO2, and heat to 80-90 C. Then test the resulting slush with pH test paper. If
it shows acidic, then bingo! MnO2 catalyzes its decomposition into K2SO4, H2SO4 and O2.
[Edited on 23-3-2023 by ave369]
Smells like ammonia....
|
|
|
Gammatron
Hazard to Others
  
Posts: 125
Registered: 30-8-2022
Location: Abandoned Uranium Mines
Member Is Offline
|
|
I dont see where it says that but it definitely releases a lot of O2 from burn tests and the white smoke condensed onto the upper end of the test
tube. Seems like the melting temp is a good bit higher than 100C but maybe it just takes a lot to get it up to temp like water.
I think that has to be it, it matches almost every characteristic on the wiki and also looks the same as samples on google images for what its worth.
I thank everyone for their input on this, it was a fun adventure! Now to figure out what I'm going to do with the kg's I have of it lol
|
|
|
ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
You can use it to make SO3/oleum. IIRC, you add concentrated sulfuric to it and heat as much as you can. That white smoke, guess what it is.... it is
SO3.
[Edited on 23-3-2023 by ave369]
Smells like ammonia....
|
|
|
Rainwater
National Hazard
   
Posts: 936
Registered: 22-12-2021
Member Is Offline
Mood: Break'n glass & kick'n a's
|
|
Just did some guess work, have not found anything to back this up
But K2S2O8 could react with hcl solution.
Thermodynamics backs this reaction
$$K_2S_2O_8 + 4HCl \rightarrow Cl_2 + 2H_2SO_4 + 2KCl$$
$$KCl + H_2SO_4 \rightarrow HCl + KHSO_4$$
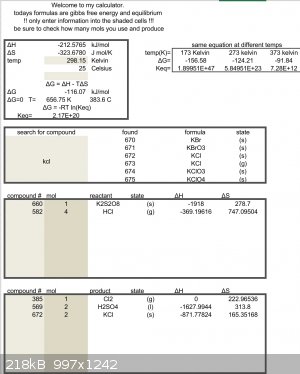
"You can't do that" - challenge accepted
|
|
|
Gammatron
Hazard to Others
  
Posts: 125
Registered: 30-8-2022
Location: Abandoned Uranium Mines
Member Is Offline
|
|
ave369, I didn't see your comment about the MnO2 test before I made my last post, I will do some more tests on it in the morning. I've spent enough
time on this today lol
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
Didn't you also heated it and found it dec into all gasses without residue? Do K2S2O8 do that too?
|
|
|
ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Gammatron  | | ave369, I didn't see your comment about the MnO2 test before I made my last post, I will do some more tests on it in the morning. I've spent enough
time on this today lol |
I've just run the test with my own sample that is definitely K2S2O8. The test was a success, the universal indicator shows pH 4, litmus paper turns
red.
Quote: Originally posted by fusso  | | Didn't you also heated it and found it dec into all gasses without residue? Do K2S2O8 do that too? |
This is what confuses me. As far as I know, K2S2O8 does not do that, but (NH4)2S2O8 does, though solubiluty does not match.
[Edited on 24-3-2023 by ave369]
Smells like ammonia....
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I don't think it is K2S2O8. I myself have this compound, and it is a strong, but sluggish oxidizer. It is capable of oxidizing silver(I) ions to
silver(III), which is impressive, but on the other hand, when it is mixed with a solid fuel (e.g. sulfur, red phosphorus, sugar) and ignited, then it
is a really lousy oxidizer. Just smouldering, no vigorous reaction at all.
If you have silver nitrate, a very good test for persulfate ion is the following:
- dissolve a little AgNO3 in 50% HNO3, or in a mix of NH4NO3 and 70% H2SO4
- Add a pinch of the material to be tested for peroxodisulfate to the solution and swirl
If you have peroxodisulfate in your sample, then you get a deep brown solution of silver(III) in the concentrated acid. If you dilute this solution
with a lot of water, then the silver(III) hydrolyses and Ag2O3 is formed, which almost immediately decomposes to oxygen and black solid Ag2O2, which
is a mixed silver(I)/silver(III) oxide.
Another good test for peroxodisulfate is this:
- Prepare a solution of sodium hydroxide or potassium hydroxide
- Prepare a solution of nickel sulfate, or nickel nitrate (not the chloride).
- Mix the two solutions: You get a pale green precipitate of hydrous Ni(OH)2.
- Prepare a solution of the solid to be tested for peroxodisulfate (heating may be necessary)
- Add the solution to the liquid with the solid hydrous Ni(OH)2 in it.
If the solution contains S2O8(2-) ions, then immediately you get a black precipitate of NiO2, which contains nickel in opxidation state +4. Again,
this test is quite specific. Only hypochlorite is capale of a similar reaction, other oxidizers like chlorate, perchlorate, nitrate, do not oxidize
the nickel (II).
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Maybe your oxidizer is NH4ClO4? This is soluble in water, but not exceptionally well, although it dissolves in water more easily than KClO4. On
heating, this decomposes, giving a yellow and fuming gas mix. The yellow color is caused by a mix of Cl2 and a little NO2, and the fuming is due to
formation of HCl and water vapor. The decomposition reaction leads to a whole bunch of compounds (Cl2, HCl, H2O, NO2, NO, N2, O2).
I have some NH4ClO4, it is not a free flowing powder, but it also is not overly hygroscopic. It is my only acceptable source of perchlorate ions
besides the very expensive HClO4, because KClO4 and NaClO4 are not available in the EU anymore. In mixes with fuels it can burn vigorously, but not as
nice as with KClO4. For me, that is no issue, I don't do pyrotechnics, my only application is using it for making interesting metal complexes, with
perchlorate as inert counterion.
Try adding a little of your solid oxidizer to solid NaOH, mix the solids, and add a drop of water. If you smell ammonia, then it most likely is
NH4ClO4.
[Edited on 24-3-23 by woelen]
|
|
|
Gammatron
Hazard to Others
  
Posts: 125
Registered: 30-8-2022
Location: Abandoned Uranium Mines
Member Is Offline
|
|
Unfortunately I don't have any nickel or silver compounds. I attempted the MnO2 test with a random amount of reagents, heated to just under a boil and
there was no ph change. It also does not react with NaOH, or any other reagent I have tried and the only one it dissolves in is hot water. Other than
being a really good oxidizer it seems chemically inert. I was also convinced that it was a potassium compound because of the purple tint of the flame,
are there other cations that can make said color?
|
|
|
MadHatter
International Hazard
    
Posts: 1346
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
Nitrate
KNO3 has a sweet smell to it. If you dare give it a taste test this is what you
should expect: At 1st it will taste cool then turn bitter. It's the 1st test I give to
comps made by people that fail to detonate in salutes.
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|
ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Gammatron  | | Unfortunately I don't have any nickel or silver compounds. I attempted the MnO2 test with a random amount of reagents, heated to just under a boil and
there was no ph change. |
For how many minutes did you keep the temperature?
Smells like ammonia....
|
|
|
Gammatron
Hazard to Others
  
Posts: 125
Registered: 30-8-2022
Location: Abandoned Uranium Mines
Member Is Offline
|
|
When I burned it with sugar it smelled like KNO3 rocket candy but maybe that's just the sugar I smelled. It had almost no taste at all, probably due
to its insolubility but what little there was tasted kinda like sodium bicarbonate.
I only heated for a few seconds in a test tube. Sadly I'm at work until the end of the day so I'll have to wait a while before I can try again.
|
|
|
ave369
Eastern European Lady of Mad Science
   
Posts: 596
Registered: 8-7-2015
Location: No Location
Member Is Offline
Mood: No Mood
|
|
Keep it for two or three minutes to perform the test properly.
Smells like ammonia....
|
|
|
MadHatter
International Hazard
    
Posts: 1346
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
Nitrate
Probably not a nitrate then. The cool/bitter taste happens with every nitrate I've
ever tasted. As for solubility most nitrates, with some exceptions such as the
barium salt, are very soluble in hot water. For the potassium salt the solubility
range, in 100 ml water, runs from 13.3g at 0C to 246g at 100C. I used to obtain
potassium nitrate in the metathesis reaction of sodium nitrate and potassium
chloride both obtained at a garden shop. These days it's just cheaper to buy it
from a reliable supplier.
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|
Sir_Gawain
Hazard to Others
  
Posts: 457
Registered: 12-10-2022
Location: [REDACTED]
Member Is Offline
Mood: Stable
|
|
Quote: Originally posted by Gammatron  | When I burned it with sugar it smelled like KNO3 rocket candy but maybe that's just the sugar I smelled. It had almost no taste at all, probably due
to its insolubility but what little there was tasted kinda like sodium bicarbonate.
I only heated for a few seconds in a test tube. Sadly I'm at work until the end of the day so I'll have to wait a while before I can try again.
|
Tasting unknown compounds has not been standard procedure since the 1700's (for good reason!).
“Alchemy is trying to turn things yellow; chemistry is trying to avoid things turning yellow.” -Tom deP.
|
|
|
Rainwater
National Hazard
   
Posts: 936
Registered: 22-12-2021
Member Is Offline
Mood: Break'n glass & kick'n a's
|
|
When stuck I make list and think out loud.please correct any mistakes ive made
Observations
Slightly solubility in cold water
Low but Increased in hot water
Insoluble in ethonol
Insoluble in acetone
Decomposes upon heating into O2 ( needs clarification. Did it relight a splint?)
Decomposes into white fumes which solid at room temp.
Leaves no residues after thermal decomposition in air
No visible reaction with 96% H2SO4 at stp
Reaction at +180c when mixed with sulfuric acid.
Documented experments and observations
Distillation with unknown + sulfuric acid producing distillate @ 180c-210c
foaming upon heating
yellow fumes observed
Distillate has low ph, no smell
Distillate does not react with copper metal
Distillate reacts with aluminum metal
combustion of unknown and sugar
unknown + H2SO4 + Cu = No reaction
energetic reaction: 5:3:2 - unknown:Al:S
flame causing melting/decomposition
Impact causes explosive decomposition
Unknown + HNO3 = no reaction
The exact values would be a great asset to this problem
Melting point ?
Decomposition temp?
Density?
Any reaction between unknown and HCl?
Not sure what your capabilities are
Density will be the easiest to measure if you have a volumetric flask and scale
Weigh the dry flask.Throw in some powdered unknown. Weigh again.
Add water and mix to remove air bubbles. Top off to the fill line and weigh again.
Density = weight of the powder ÷ (size of the flask in ml - weight of the water added
In grams)
Can you tell us more about the distillate collected.
Density, boiling point.
you mentioned a ph 4. How much base to bring a known volume to netural?
"You can't do that" - challenge accepted
|
|
|
| Pages:
1
2 |