| Pages:
1
2
3
4 |
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
Snailsattack, I think you forgot to include the extra volume when calculating solubility.
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by teodor  | There are 2 reasons why some sources contain incorrect data for CuSO4.
1. Solubility curve of CuSO4 has 3 anomalies in the pentahydrate range: 28.9, 34.85, 53.72C. It is like spikes where properties of crystalls are
changing very fast in very narrow range (fractions of degree) and comes back to the curve:
[]
There is a reason for this behaviour: water molecules in crystalls change behaviour from oscilation to rotation. But those anomalies of CuSO4
solubility were discovered in the end of 19 centure. Try to evaluate the quality of your in-house methods. And probably what you can observe at those
points is "there is no constant equilibrium". |
Why would it even do this? There's only 15 data points on that graph. Can the anomalous 3 spikes be attributed to some sort of weird error? Were the
measurements repeatable? Each data point is between 1 and 3 degrees apart, so how wide are the spikes?
I've never seen a graph showing this behavior or read anything about it. It's also not reflected in the table you cited.
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
What extra volume? Solubility is supposed to be measured as grams per liter of solvent rather than grams per liter of solution.
If you measure in g/L of solution you have to know the density of the solution and that's a pain in the butt. I don't think that reframing the
measurements in g/L of solution fixes the problem.
|
|
|
teodor
National Hazard
   
Posts: 924
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
Quote: Originally posted by Sulaiman  | Maybe not a miracle but certainly a revelation.
Two obvious questions:
1 is this common behaviour for hydrated ions/molecules?
(eg MgSO4, Cu(NO3) 2 etc)
2 can I use this effect to create a hyper-saturated copper sulphate solution ? |
1. This discovery is connected with the name of Linus Pauling. He wrote several excelent books by the way, for example: "Quantum Mechanics", "The
nature of the chemical bond" which are highly recommended though require refereshing of mathematical knowledge.
And really, following his prediction, that behaviour was discovered in many compounds.
Attachment: taylor1936.pdf (686kB)
This file has been downloaded 190 times
2. I agree that it would be interesting to utilize somehow this behaviour in chemical demonstration. This definitely can affect crystall grow. As for
supersaturated solution I think it is easier to try some hardly crystallisable anions.
[Edited on 12-3-2023 by teodor]
|
|
|
teodor
National Hazard
   
Posts: 924
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
It is. Look at 2 different values printed at 20C and 54C. That probably because author was unable to get one defined value.
[Edited on 12-3-2023 by teodor]
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
Why "Solubility is supposed to be measured as grams per liter of solvent rather than grams per liter of solution" and not the reverse?
The solute is gonna dissolved in solvent to form the final solution. Why is molaRity unimportant? That's what we ultimately want to know, isnt it?
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by teodor  | This discovery is connected with the name of Linus Pauling. He wrote several excelent books by the way, for example: "Quantum Mechanics", "The nature
of the chemical bond" which are highly recommended though require refereshing of mathematical knowledge.
And really, following his prediction, that behaviour was discovered in many compounds. |
Alright, the graph in that paper is reasonably convincing. The spike at 53.7°C is about 3°C across, which might be wide enough to test for. It could
be referenced against the solubility at ~47°C and ~61°C.
|
|
|
yobbo II
National Hazard
   
Posts: 765
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
What are the units on the left hand side of the graph?
I presume it's degrees centigrade along the bottom (as Teodor said)
Yob
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by yobbo II  |
What are the units on the left hand side of the graph?
I presume it's degrees centigrade along the bottom (as Teodor said)
Yob |
which graph
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by teodor  | Quote: Originally posted by Texium  |
I was typing out a longer response last night, but I lost it by accident, and teodor and Amos pretty much covered what I was going to say.
|
Oh, yes, it happend for me several times already. Now I always copy responses to an external text editor when they become long and continue there.
I think there is a nice demo illustrating your and Amos idea "there are a lot of variables some of which are unknown".
https://en.wikipedia.org/wiki/Storm_glass |
The important thing about storm glasses is that they never actually worked.
|
|
|
yobbo II
National Hazard
   
Posts: 765
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
http://www.sciencemadness.org/talk/files.php?pid=682245&...
The graph at the above link.
What are the units on the left hand side of the graph?
I presume it's degrees centigrade along the bottom (as Teodor said)
There is another paper on H2O Copper sulphate system attached.
There is no talk of strange spikes?
It's all rather technical.
Yob
Attachment: CHEM_Sibarani_et_al_Critical_Evaluation_2022_Chemical_Engineering_Science.pdf (3.1MB)
This file has been downloaded 223 times
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Before I recuse myself from this thread to preserve my
sanity, let me point out a couple assumptions you've made that will make roundin' off those corners hard if not impossible for you...
Quote: Originally posted by SnailsAttack  | | 24 hours? I give it five minutes max before a sample of solid copper sulphate emersed in water is effectively fully equilibriated with the solution.
|
yobbo II already addressed this, but you never replied, so I feel it's worth repeating. This is going to be
a big problem for you, and it makes me wonder if you've ever actually tried to make a saturated copper sulfate solution before, cause it tends to take
a lot longer than 5 minutes to get everything dissolved, even with heating and stirring, not just immersing the solid in water. You're not going to
get close to saturation if you only wait 5 minutes. It'll depend on how large the crystals you're trying to dissolve are, too.
Quote: Originally posted by SnailsAttack  | | I've observed that anhydrous copper sulphate rehydrates instantly on contact with water, which raises more questions about the semantics of what the
solubility of anhydrous copper sulphate actually means if it can't coexist with water. |
Yes, it turned blue,
wonderful, but do you know for sure that it's immediately forming the pentahydrate? Based on observations that I have made before, I don't think that
it is. If you add 5 equivalents of water to 1 equivalent of anhydrous copper sulfate and thoroughly mix it, it doesn't fully absorb all the water and
immediately become a nice homogeneous pentahydrate. This won't affect the final outcome, but it will change how long it takes X moles of anhydrous
copper sulfate to dissolve in Y+5X moles of water, compared to how long it would take X moles of pentahydrate to dissolve in Y moles of water.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by SnailsAttack  |
What extra volume? Solubility is supposed to be measured as grams per liter of solvent rather than grams per liter of solution.
If you measure in g/L of solution you have to know the density of the solution and that's a pain in the butt. I don't think that reframing the
measurements in g/L of solution fixes the problem. |
Do some more reading and some more calculations until you get it, because as fusso pointed out: that calculation where you get a 10% difference on
page 2 is wrong.
Do the calculations again and you will see "reframing" solves your problem.
Solubility is measured in grams per total volume, not grams per amount of solvent. Have you ever made a solution in a chemistry class? You will add an
amount of compound to a volumetric flask and add solvent till you reach a certain volume.
And why would you have to know any density to calculate or express any solubility this way? You were the only one in this thread who needed a
density in any calculation. You only need density of you would want to know the total weight of the solution. Which is not relevant for solubility.
[Edited on 12-3-2023 by Tsjerk]
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Good call, this thread makes my head hurt.
Quote: Originally posted by Texium  |
Quote: Originally posted by SnailsAttack  | | 24 hours? I give it five minutes max before a sample of solid copper sulphate emersed in water is effectively fully equilibriated with the solution.
|
yobbo II already addressed this, but you never replied, so I feel it's worth repeating. This is going to be
a big problem for you, and it makes me wonder if you've ever actually tried to make a saturated copper sulfate solution before, cause it tends to take
a lot longer than 5 minutes to get everything dissolved, even with heating and stirring, not just immersing the solid in water. You're not going to
get close to saturation if you only wait 5 minutes. It'll depend on how large the crystals you're trying to dissolve are, too. |
Yeah, in retrospect I think 5 minutes isn't long enough. Maybe I'll do a separate test to see how long it takes before the saturation hits a limit.
Quote: Originally posted by SnailsAttack  | | Yes, it turned blue, wonderful, but do you know for sure that it's immediately forming the pentahydrate? Based on observations that I have made
before, I don't think that it is. If you add 5 equivalents of water to 1 equivalent of anhydrous copper sulfate and thoroughly mix it, it doesn't
fully absorb all the water and immediately become a nice homogeneous pentahydrate. This won't affect the final outcome, but it will change how long it
takes X moles of anhydrous copper sulfate to dissolve in Y+5X moles of water, compared to how long it would take X moles of pentahydrate to dissolve
in Y moles of water. |
You're right, it's possible that it doesn't rehydrate immediately to the pentahydrate even in direct contact with water. For instance, I've observed
that calcium sulphate is reluctant to form the dihydrate. That's another matter though.
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
nope
Really? This wiki page suggests that the standard units are grams per 100 milliliters of water. Very few sources actually specify whether it's grams per
unit solvent or grams per unit solution, however.
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by SnailsAttack  |
Really? This wiki page suggests that the standard units are grams per 100 milliliters of water. Very few sources actually specify whether it's grams per
unit solvent or grams per unit solution, however. |
Yeah, all the readings I can find on solubility say that “g/mL” or “g/L” refer to grams per unit of solvent, not grams per unit of solution.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Well, that Wiki page is a bit ambiguous, probably because the purpose of that sentence is explaining water is the solvent, not what is meant by g/L.
Molarity and g/L are interchangeable by knowing only molecular weight.
Care to share some of those readings?
https://www.omnicalculator.com/chemistry/molarity
[Edited on 13-3-2023 by Tsjerk]
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by Tsjerk  | Well, that Wiki page is a bit ambiguous, probably because the purpose of that sentence is explaining water is the solvent, not what is meant by g/L.
Molarity and g/L are interchangeable by knowing only molecular weight.
Care to share some of those readings? |
Yeah, here's some I found by just googling: 1 2 3 4 5
measuring in grams per liter solvent is easier than grams per liter solution because, like I said, maths with the latter requires you to know the
density of the solution which requires additional measurements.
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by yobbo II  |
There is another paper on H2O Copper sulphate system attached.
There is no talk of strange spikes?
It's all rather technical. |
The graph on page 11 looks.. very clean. I might convert that to a table.
Looks like the authors investigated a ton of different sources. If they came across any that included the spikes, they were apparently rejected.
Admittedly I'd love to just ignore that study for the sake of simplicity, but that would be bad science.
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by SnailsAttack  | Yeah, here's some I found by just googling: 1 2 3 4 5
measuring in grams per liter solvent is easier than grams per liter solution because, like I said, maths with the latter requires you to know the
density of the solution which requires additional measurements. |
Here’s the thing: that method of
measurement is only useful for knowing the maximum solubility of a substance at a given temperature, i.e. what you are trying to do. In nearly every
other application, it is more useful to know g/mL solution, or molarity, because you can measure out any volume of the solution and know how much mass
is dissolved in that portion. If you had made up the solution as grams/mL solvent, you’d either have to measure the total volume of the solution to
be able to do the same, or weigh the solution and measure everything by mass, but either way it’s a pain in the ass and completely unnecessary. So
yes, for YOUR purpose, g/mL solvent is easier because you aren’t actually doing anything with the solution you make. If you were using the solution
in a reaction or for some spectroscopy experiment, then you would be the one doing extra measurements and math, while someone who prepared using g/mL
solution would be all set.
I will reiterate that most chemists simply do not care about the maximum solubility of a substance because it is completely irrelevant unless you are
growing crystals or doing some esoteric physical chemistry like some of the papers posted in this thread.
|
|
|
yobbo II
National Hazard
   
Posts: 765
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
At snailsAttack.
You probably answered your own question in the opening question, thus:
"They had trouble getting or identifying a pure pentahydrate......."
edit:
Posssibly not after reading some more on sci.mad!
Paper attached on the dehydration of Copper Sulphate.
Extract:
A study of the thermal transitions in copper sulphate pentahydrate has been made from
warming curves obtained by a differential thermocouple method. Small transitions were
observed at 29°C, 35°C, 53. 7°C, and dehydrations at 96.5°C, 102°C, and at 113°C. The first
three of these may be interpreted as transitions from oscillation to rotation of the water molecules
in the crystal. The last three are associated with the stepwise dehydration to the tetra-,
trio, and monohydrates, respectively. The existence of the tetrahydrate, not previously known,
has been demonstrated. A discussion of the dehydration in the light of its crystal structure is
given. A mechanism of dehydration by heat, based on the concept of molecular rotation, has
been suggested.
Graph from above is below:
It has nothing to do with actual solubilities IMHO
See attached file with similar bumps on a graph of heat absorbtion.
Another edit:
Having done some reading on sci.mad. this subject has been doing the rounds for quite some time.
Would there be a hysteresis error?
One thing that may be causing a difference in stating the solubility of different hydrates (I don't know myself) is the way the solution is actually
taken to saturation.
You can add your salt (whatever hydrate) to the water and heat up to the measureing temperature and wait for everything to dissolve and then measure
the liquid part for m/l or grams per mole water (or whatever).
You could also heat up the water + salt untill everything is dissolved and then slowly cool untill you see the first crystal form (a messy method
IMO).
You are now approaching saturation 'from the other side' as it were.
I never heard this mentioned anywhere so it is probably not a problem.
Yob
Attachment: dehyd_OF_cu_sulfate.pdf (1.8MB)
This file has been downloaded 195 times 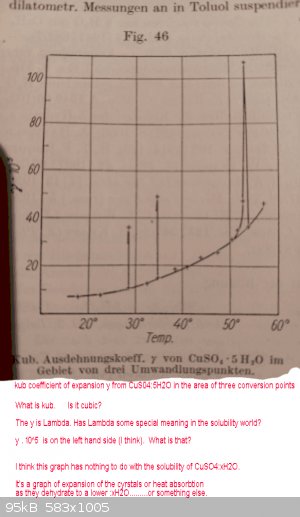
[Edited on 13-3-2023 by yobbo II]
[Edited on 13-3-2023 by yobbo II]
|
|
|
teodor
National Hazard
   
Posts: 924
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
" It is like spikes where properties of crystalls are changing very fast in very narrow range (fractions of degree) and comes back to
the curve". I didn't say it is the solubility.
The solubility is dependent on the parameter depicted on the graph.
The necessary condition of thermodinamic equilibrium is "stability against small perturbations" - https://en.wikipedia.org/wiki/Thermodynamic_equilibrium#Stab...
But we can expect the steepness of the graph is related to steepness of solubility changes if we would able to measure it in this small temperature
range.
The concept of existing (=constant) equilibrium implies we could find a method how to put the system in this state otherwise we are talking about some
virtual property of the model which has no relation to the real life.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I should have listened to Texium. I don't care whether it is gram per liter solvent or per volume anymore. Good luck with it. All I know is that the
initial hydration doesn't influence the final solubility.
My god, just use molarity. I have worked in labs for years, never ever have I doubted grams not being per volume. Never have I seen people adding a
volume of solvent to a weight of a to be solute.
Saturated solutions you have on hand by having a bottle with a thick layer of solid on the bottom of the bottle, and even then; who cares how much is
actually dissolved.
[Edited on 13-3-2023 by Tsjerk]
|
|
|
yobbo II
National Hazard
   
Posts: 765
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
I understand now.
Probably everyone in the thread knew that except Yobbo!
|
|
|
SnailsAttack
Hazard to Others
  
Posts: 166
Registered: 7-2-2022
Location: The bottom of Lake Ontario
Member Is Offline
|
|
Quote: Originally posted by yobbo II  | You probably answered your own question in the opening question, thus:
"They had trouble getting or identifying a pure pentahydrate......."
|
I found that copper sulphate forms a stoichiometric pentahydrate when crystallized from solution under normal conditions, so its use shouldn't present
an issue, although a measurement procedure involving the crystallization of other hydrates at high temperature would get messy.
The dissolution process probably doesn't lag too much (solid crystals should equilibrate with solution relatively quickly), but the crystallization
process could definitely lag, resulting in supersaturation, hence why I based my method of measuring solubility on the former process.
Quote: Originally posted by yobbo II  | One thing that may be causing a difference in stating the solubility of different hydrates (I don't know myself) is the way the solution is actually
taken to saturation.
You can add your salt (whatever hydrate) to the water and heat up to the measureing temperature and wait for everything to dissolve and then measure
the liquid part for m/l or grams per mole water (or whatever). |
Yeah, pretty much exactly my process.
Quote: Originally posted by yobbo II  | You could also heat up the water + salt untill everything is dissolved and then slowly cool untill you see the first crystal form (a messy method
IMO).
You are now approaching saturation 'from the other side' as it were. |
Yep.
|
|
|
| Pages:
1
2
3
4 |