Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
2,4-DinitrophenylHydrazine Synthesis [Lab Report]
Synthesis of 2,4-Dinitrochlorobenzene
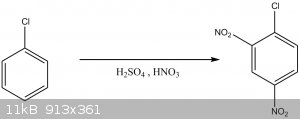
First a Sulfo-Nitric solution was prepared by carefully mixing 30mL of sulfuric acid [1,8 g/mL] and 14,5 mL of azeotropic nitric acid, then the
solution was left to cool.
With vigorous stirring, to the sulfo-nitric mixture, 8mL of Chlorobenzene were added by portions, not letting the temperature go above 50ºC. Once all
the chlorobenzene was added the mixture was heated to 95ºC and maintain for 2hs with stirring.
After the 2hs passed, to the solution, 150mL of water were added and stirred, the mixture separates in 2 layers. The organic phase was washed with
water 3 times.
Crud Yield 13,01g [81,4%]
![2,4-DNCB [I].JPG - 280kB](https://www.sciencemadness.org/whisper/files.php?pid=681971&aid=97381) ![2,4-DNCB [II].JPG - 252kB](https://www.sciencemadness.org/whisper/files.php?pid=681971&aid=97383)
Synthesis of 2,4-Dinitrophenyl hydrazine.
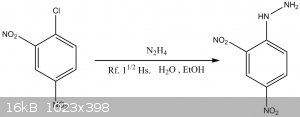
These syntheses requires the preparation of a Hydrazine free-base solution.
Preparation of Free-base Hydrazine solution:
On a beaker, 7g of Hydrazine sulfate and 25mL of water were mixed, and the mixture was heated to boiling. Once boiling was observed, the heat was
turned down and 15g of anhydrous sodium acetate were added in portions. Once all the acetate was added the solution was left hot for 5min and then
15mL of ethanol were added and the mixture filtered, with the filtered washed twice with hot ethanol. The solution was stored for immediate use.
2,4-Dinitrophenylhydrazine:
On an Erlenmeyer flask with ground glass joint, 10g of 2,4-Dinitro-chlorobenzene were dissolved in 50mL of 95% Ethanol, to which the hydrazine
solution is added. The mixture is left to reflux for 1 ½ Hs.
The mixture was then cooled, filtered, and the precipitated product recrystallized with butanol [30mL per gram of 2,4-Dinitrophenylhydrazine]
Yield: 4.03g [41,2% based on Dinitrochlorobenzene]
![2,4-DNPH [I].JPG - 290kB](https://www.sciencemadness.org/whisper/files.php?pid=681971&aid=97385) ![2,4-DNPH [II].JPG - 277kB](https://www.sciencemadness.org/whisper/files.php?pid=681971&aid=97387)
Source: http://www.orgsyn.org/demo.aspx?prep=CV2P0228
|
|
|
charley1957
Hazard to Others
  
Posts: 168
Registered: 18-2-2012
Location: Texas
Member Is Offline
Mood: Beginning to cool off
|
|
Nicely done, and a nice write up. Beautiful red-orange crystals. Now what are you going to do with this stuff?
You can’t claim you drank all day if you didn’t start early in the morning.
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Well done!!! Useful reagent for analysis of ketones and aldehydes (Brady's reagent).
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
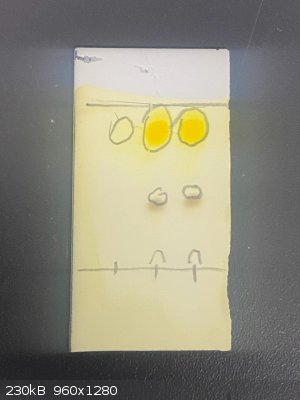
Funny enough, I actually just used some as a stain today! It’s especially useful when, as in my example, the starting material and the product have
the same Rf and are thus indistinguishable by UV visualization alone:
|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
Thanks Charley1957 and Fery.
Yup, i use these compound to make Brady's Reagent and identify Ketones and Aldehydes 
Nice TLC method there Texium, i haven't heard of that one before.
[Edited on 1-3-2023 by Chem Science]
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by Chem Science  | Yup, i use these compound to make Brady's Reagent and identify Ketones and Aldehydes 
Nice TLC method there Texium, i haven't heard of that one before. |
Well, it’s the same principle as the
Brady’s reagent test, just with staining a TLC plate instead of doing the test in solution. In this case, my starting material is a benzyl bromide
and my product is an aldehyde.
|
|
|