Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Perilla plant extract & lemon juice yields magenta compound
My wife planted one of these plants of the Perilla family a few years ago and now it grows everywhere in the garden.

The Vietnamese (and other Asians) use this plant in many ways for cooking, but what intrigued me from a chemistry side is the color change when used
as a tonic drink.
The leaves are first washed in cold water:

They are then placed in a cup and boiling water added. This gives a pale colored liquid. A lemon or lime is then squeezed into the liquid, which
results in an immediate color change to magenta. See below video.
Attachment: 3 Colorchange.MOV (3.5MB)
This file has been downloaded 165 times
I was interested see if this magenta color would result in a similarly colored dry solid. So I placed some in a shallow dish:

It dried slowly, and now after a month I do indeed have a magenta solid, still very slightly sticky. And with mold growing on it. Quite a pleasant
smell.
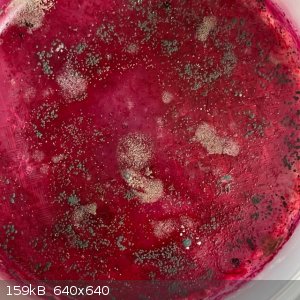
I would love to know what the magenta compound is. And maybe this is a safe demo for school students to show a color change?
|
|
|
Parakeet
Hazard to Self
 
Posts: 73
Registered: 22-12-2022
Location: Japan
Member Is Offline
Mood: V (V)
|
|
Perilla contains a pigment called anthocyanin. Anthocyanin is also contained in many other plants such as hydrangeas, red cabbages and pansies.
And yes, they are great materials for teaching. Here is a video that Japanese TV station made for education. https://www2.nhk.or.jp/school/movie/clip.cgi?das_id=D0005400... It’s Japanese but I think you can understand the pictures.
I haven’t done this experiment with perilla before, but I have with red cabbages. Keep in mind that they are unstable and not suitable for storage.
I also like to eat perilla very much! I love it’s nice smell.
|
|
|
Rainwater
National Hazard
   
Posts: 847
Registered: 22-12-2021
Member Is Offline
Mood: indisposition to activity
|
|
https://en.m.wikipedia.org/wiki/Anthocyanin
anthocyanins
Very nice color, the change was a result of acidity, add a base and it will turn blue
"You can't do that" - challenge accepted
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Thanks for the comments! I will play around with acidity and see which colors I get.
|
|
|
Liamatpm
Harmless

Posts: 48
Registered: 7-12-2016
Location: Maine
Member Is Offline
Mood: Using the process of cellular respiration to stay (barely) alive
|
|
Hmm that is pretty interesting. The colors are rather nice
|
|
|
Bedlasky
International Hazard
    
Posts: 1225
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Lion850, use sodium carbonate as a base. From my experience NaOH destroy anthocyanins rather quickly, they turn brown and lose their ability to change
colour with pH.
|
|
|