| Pages:
1
2 |
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Extraction of caffeine from tea or coffee!
Caffeine is found both in tea and coffee, IIRC to a greater extent in tea per gram of material.
The method of extraction depends on its solubility in chloroform, CHCl3.
The extraction from tea is preferred as emulsions form less readily, and as there are less coloured impurities.
Protocol:
1. Weigh out 50 g tea, or coffee (roast ground), or 20 g of instant coffee, and warm in a beaker with 200 ml H2O.
2. Boil this gently for 15 minutes.
3. Remove solids by filtering, and wash with a little hot H2O.
4. Heat the filtrate to boiling, and add 100 ml of 0.3 M lead II acetate (have a look at the PbO2 thread for details) in order to precipitate organic
acids and proteins such as albumin.
5. Filter a small sample of this, and take the clear filtrate, and add lead acetate once again - this step is done to ensure that all has been
precipitated.
6. Filter the whole filtrate, and add 2 M sulphuric acid to the filtrate to precipate the lead acetate (forming PbSO4). Filter this again.
7. Add 2 M ammonia (or whatever concentration you have) until it is at neutral pH, and then boil down to 100 ml.
8. Allow to cool a little, and add 5 g of decolorising charcoal and bring to boil again. Filter as before to remove the coal.
9. Add one portion of CHCl3 (40 ml) , and invert frequently (even better is to put it on rollers), rather than shaking, to avoid emulsification.
10. Repeat 9.
11. Add Na2SO4 (anhydrous) to the combined chloroform extract to remove traces of water, then filter.
12. Distill off the trichloromethane (waterbath), then evaporate to dryness. Dont do this in the house, do it outside. Recrystallise from hot water.
Yield : about 1 gram. A single cup of coffee contains about 110 mg (less in instant coffee, more in freshly ground coffee http://www.cspinet.org/new/cafchart.htm ).
Don't forget, this is a HUGE yield considering the original mass (50g) for a biological product!
Notes:
Instead of the chloroform, I am sure trichloroethylene can be used, which is a common solvent in hardware stores.
This procedure can be upscaled of course. Just get i.e. 500 g of tea, or coffee, and you will get about 10 g of caffeine. Enough to keep you energised
for a while  - not that I am suggesting to eat this (thats my disclaimer) - not that I am suggesting to eat this (thats my disclaimer)
In this prep, I wouldnt be worried about the lead, because it doesnt dissolve in the chloroform/trichloroethylene. Not even a nanogram. Plus, if you
do the recrystallisation as advised, you will remove any of the chlorinated solvents themselves by the recrystallisation procedure
(chloroform/trichloroethylene are not soluble in water). In addition, a bit of heat evaporates these.
Although I am sure there are ways to poison yourself with it, I am equally sure if you follow the procedure there won't be a problem. That is,
if you don't eat a gram of caffeine to test its effects (always start with very little, i.e. 100 mg in this case).
I am not trying to turn this into some drug discussion at all, I just felt there may be some individuals who are more than willing to test this with
some rather excessive amounts 
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Organikum
resurrected
    
Posts: 2339
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
Sounds easier than a extraction with supercritical CO2 at least.... 
But for what good should the extracted coffeine be?
Hm, what about cannabinol extraction from low-THC hemp? This cannabinol is of course not psychoactive but damned legal.
At least until one converts it to THC-9 with EtOH and H2SO4.......
....what nobody would do of course.
Because its not legal.
ORG
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
I've did this from tea, but following a different procedure, which was a bit easier IMHO.
I don't remember it exactly, but it was something like this:
Reflux the tea leaves with CaCO3. The tannins and gallic acids present will precipitate als calcium salts and can be filtered off.
The remaining solution is then extracted with 4 portions of DCM which is allowed to evaporate or distilled off.
This will yield very crude caffeine. According to the text the caffeine could be further purified by recrystallization from acetone/ligroin and
sublimation.
Purity check involves forming the salicylate salt which apparantly has a very sharp melting point.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Reverend Necroticus Rex
Hazard to Others
  
Posts: 196
Registered: 15-6-2004
Location: Right behind you.......
Member Is Offline
Mood: Poisonous
|
|
I read once about it being possible to sublife caffeine by mixing a teaspoon or so of tea with a little MgSO4, make a paste with a few drops of water
and simple heat in a test tube, apparently the caffeine just sublimises off ans gets left at the top of the tube.
The sun is shining on a brand new day
Blackened corpses burn where they were slain
Self-flagellation prompts him to confess, Bless me father, for I made this mess.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
| Quote: |
But for what good should the extracted caffeine be? |
One option is to eat it 
Or, if you are into heterocyclic chemistry, you can use this as a precursor for more heterocyclics...you can nitrate it, acetlylate it, whatever you
wish 
But most importantly you can say: I know how to extract caffeine, here I got a few grams! Just like this silly pride you feel when you first time ever
make a tetraamine complex, and the colour deepens... there is no direct use for it, still its cool!
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Disgard the cafein and keep the now clean tea or coffie....
You can then prepare and drink homemade cafeinfree tea or coffie         
JUST KIDDING FOR THOSE WHO THINK I'M SERIOUS ABOUT THIS.
[Edited on 1-7-2004 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
for a minute..... I was wondering... 
Oh, and Org,.. it is possible to extract THC from the leaves... with acetone it worked nicely. Left a nice sticky mass.. that had a very
characteristical smell to it. Needless to say, that was a proof of principle only 
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Chemoleo,
In place of toxic aceton I would use less toxic anhydrous Ethanol.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Oh... but... why would I worry about toxicity, if I didnt eat/smoke it? Plus... the acetone evaporates after a few days...
Unless THC reacts with acetone... I didnt check the structure to see whether it's possible.
Anyway...
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Just a try to have cafein jpeg
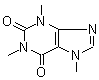
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
And now THC...
great I didn't notice yet it was possible to make attachements..this will help a lot....
There is no reason for aceton to react since cannabisol (-binol) doesn't contain any amine group...it is one of the few psychoactive molecule
that doesn't have an amine (nitrogen atom).
[Edited on 6-7-2004 by PHILOU Zrealone]
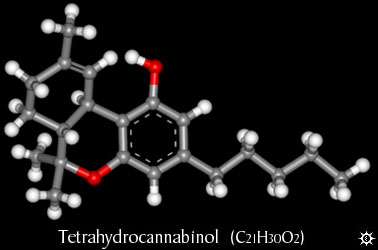
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Caffeine is a partly saturated purine derivative, consisting of a 6-membered ring with 2 N's fused to a 5-membered ring with 2 Ns, with CH3s on
the 1, 3 and 7-N's, and keto O's on the 2 and 9-C's. It is, as the result, a diketone with 4 tertiary-amine N's. I believe it is
degraded to purine by body metabolism before being excreted.
The 6-membered ring part has some similarity to barbituric acid. This opens up the possibility of converting caffeine to barbiturates, but this is not
the standard industrial method of synthesis (urea reacted with diethyl malonate in presence of sodium ethoxide).
John W.
|
|
|
mericad193724
Hazard to Others
  
Posts: 121
Registered: 4-6-2006
Location: New Jersey, USA
Member Is Offline
Mood: why do you care?
|
|
Hello,
Would this modified method work for getting fairly pure caffeine?...
Start with 15 No-Doz caffeine pills - 200mg per tablet
Crush pills and mix with 500ml of boiling water, filter out solids
Boil for 15 minutes
Cool and add 120ml of Methylene Chloride to water mixture
invert frequently to mix (Meth Chloride and Water)
Add another 75ml (Meth Chloride) and mix again in the above manner
Add Na2SO4 (anhydrous) to remove water
Distill and evaporate Methylene Chloride until Caffeine powder is left
Efficient Yeild should be around 3g of fairly pure caffeine.
Would this method work or am I an idiot???
thanks,
mericad193724
(The amounts of liquids are "guess-timates" are they ok???)
|
|
|
lordmagnus
Hazard to Self
 
Posts: 92
Registered: 10-1-2006
Location: Webster, TX
Member Is Offline
Mood: No longer annoyed ( I found a new girlfriend)
|
|
Supercritical CO2 yields a pretty clean caffeine base to work with, that's how Folgers does it up in Houston, TX. then they sell the caffeine to
pharmaceutical companies that make VIVARIN, No_DOZE, and Caffeine Citrate (controlled substance to treat ADHD). Then they sell the decaffinated
coffee.
Hey, what do you call a COW (Bovine) that has had an abortion?
De-Caffinated... yeah I know, that was crude
I\'ll kill a man in a fair fight, or if I think he is gonna start a fair fight, or if he\'s bothrn me allot, or if I am getin payed good, or probably
over a good woman.
-Jayne Cobb (Serenity) |
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
| Quote: | Originally posted by chemoleo
In this prep, I wouldnt be worried about the lead, because it doesnt dissolve in the chloroform/trichloroethylene. Not even a nanogram.
|
Organic molecules can dissolve in organic solvents with an appropriate ligand. Add a crown ether (of the form (-CH<sub>2</sub>
CH<sub>2</sub>O-)<sub>n</sub> and you can dissolve damn
near any metal salt in a nonpolar solvent. Any molecule with exposed lone pairs like that will probably carry a metal ion into solution. Could
anything like that be present in the mixture of chemicals that make up coffee? and you can dissolve damn
near any metal salt in a nonpolar solvent. Any molecule with exposed lone pairs like that will probably carry a metal ion into solution. Could
anything like that be present in the mixture of chemicals that make up coffee?
I’m guessing that a secondary purpose of the sulfuric acid is for the protons to bind to any such ligands and release the lead as free cations,
aside from the obvious precipitation.
|
|
|
alyks
Harmless

Posts: 24
Registered: 9-5-2006
Member Is Offline
Mood: No Mood
|
|
I extracted caffeine from these "stay awake" pills. Everything was insoluble except the dextrose and caffeine. Dissolve and boil.
|
|
|
mericad193724
Hazard to Others
  
Posts: 121
Registered: 4-6-2006
Location: New Jersey, USA
Member Is Offline
Mood: why do you care?
|
|
Caffeine and dextrose both dissolve in what.... Chloroform, Methylene Chloride or Water?
thanks,
mericad193724
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
isn't dextrose like starch and not really soluble in anything?
caffeine is somewhat soluble in water and dissolve very well in chloroform, DCM, trichloroethene, ect.
I have extracted caffeine before, but they were nice brown long crystals, I didn't do a good job on purifying them, I could but why?
I also have some caffeine pills that I plan to do an extraction on, but only when I find a good chemistry use for caffeine.. really it seems like a
junky chemical when it comes to reacting with things, however I would love to extract thiobromine, or maybe turn caffeine into thiobromine but methyl
groups psh..there not really going anywhere now are they?
thiobromine is interesting IMO, it makes you happy and filled loved, that's why "they" say eat chocolate when you fill depressed.. anyway's what could
extract it? it looks very hard to do I think.
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Dextrose is another name for glucose. It's freely soluble in water. Dextrin is a gum.
[Edited on 17-6-2006 by unionised]
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
Oh alright thanks! That maks it a lot more easier. I think I am going to begin extraction of caffiene just for the heck of it.
|
|
|
lordmagnus
Hazard to Self
 
Posts: 92
Registered: 10-1-2006
Location: Webster, TX
Member Is Offline
Mood: No longer annoyed ( I found a new girlfriend)
|
|
Seems I recall that Caffeine works as fairly good developer for B&W film, oddly enough so does horse urine (same guy wrote those articles, I don't
pretent to know why he decided to try horse urine), anyway here is the link for them;
http://www.photo.net/bboard/q-and-a-fetch-msg?msg_id=003aPB
I\'ll kill a man in a fair fight, or if I think he is gonna start a fair fight, or if he\'s bothrn me allot, or if I am getin payed good, or probably
over a good woman.
-Jayne Cobb (Serenity) |
|
|
neopentane
Harmless

Posts: 1
Registered: 14-6-2006
Location: USA
Member Is Offline
Mood: No Mood
|
|
I did a caffeine extraction from tea last year using Na2CO3, a vacuum filtration setup, and MeCl2 that pulled pretty close to the theoretical yield.
Not really worth the time though after going through once for the hell of it; easier methods exist.
|
|
|
Nerro
National Hazard
   
Posts: 596
Registered: 29-9-2004
Location: Netherlands
Member Is Offline
Mood: Whatever...
|
|
Could a different metal ion be used to precipitate that crap in the solution because if I'm going to do this I really want to be rid of the Pb...
#261501 +(11351)- [X]
the \"bishop\" came to our church today
he was a fucken impostor
never once moved diagonally
courtesy of bash
|
|
|
pantone159
National Hazard
   
Posts: 590
Registered: 27-6-2006
Location: Austin, TX, USA
Member Is Online
Mood: desperate for shade
|
|
It can be done without the Pb. When I did this, I added CaO to the boiled tea extract to ppt tannins, filtered (very slow), then extracted the
caffeine with DCM. I did not follow the advice to shake the sep funnel very gently, and found myself with an annoying emulsion. I added some Na2CO3
to the mess, refiltered, and I was able to separate that. Evaporation of the DCM yielded the crude caffeine, which I have not (yet) purified further.
I'm not claiming this was the ideal procedure, but it did seem to work.
There are actually quite a lot of different procedures for caffeine extraction, I have printed copies of around 5 or so.
|
|
|
pantone159
National Hazard
   
Posts: 590
Registered: 27-6-2006
Location: Austin, TX, USA
Member Is Online
Mood: desperate for shade
|
|
P.S. I was more-or-less trying to follow the procedure in this post:
http://www.scienceforums.net/forum/showpost.php?p=202318&...
with some discussion here:
http://www.chemicalforums.com/index.php?topic=7094.0
I made the following changes: Instead of using n-propanol for the extraction, I used DCM. DCM seemed to be more selective than n-PrOH, I found that
all of my extracted material was acetone soluble, so that part of the refinement is unnecessary when using DCM.
|
|
|
| Pages:
1
2 |