| Pages:
1
2 |
Sev
Harmless

Posts: 5
Registered: 3-6-2006
Member Is Offline
Mood: No Mood
|
|
Synthesis of DDT?
After getting attacked my mosquitos yet again in my house, with the Mosquito Onslaught only beginning, I decided I wanted to try to do something
about it. I want to synthesize and paint the walls of my freaking room with DDT. On the other hand, I have zero organic chem experience.
Anyone got a rundown of the synthesis I can follow?
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
DDT is made by condensing chloral with chlorobenzene in the presence of Oleum or chlorosulfonic acid. On Lambdasyn there's a description of the
synthesis. It's actually quite straightforward when you have the chems... and getting those is the real problem.
You'd be better off by making HCH (hexachlorocyclohexane, known under various trade names such as Lindan), the chems for which are easier to get.
It is made by chlorinating benzene at its boiling point under irradiation with a strong light source and in the strict absence of friedel- crafts
catalysts such as Fe or Al. The product is a solid and crystallizes upon cooling if the synthesis was successful.
For benzene, look up the thread on it here, it is easy to make.
Chlorine too.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Oleum or chlorosulfonic acid are not really necessary, I have a method from a lab book which just uses conc sulfuric. It is attached.
Are you sure you did not mean DEET; N,N-diethyltoluamide? This would be easy as well to make, but one of the precursors, diethylamine is heavily
watched because it can be used for making LSD. You also would need toluyl chloride, but I don't think it is heavily watched.

|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
While DDT is of fairly low human toxicity, the same cannot be said of the precursors. Chloral is a controlled drug and the manufacture of DDT is
generally banned.
DEET is a much better bet.
|
|
|
Sev
Harmless

Posts: 5
Registered: 3-6-2006
Member Is Offline
Mood: No Mood
|
|
Thanks, folks, you've been a lot of help.
And, yeah, I know that manufacture of DDT is banned -- which is why I'm making it myself.  I'm used to dealing with toxic chemicals, but I'd like to try making some DDT 'cause it's essentially harmless to humans in any sane
dosage, it can be painted on surfaces and hangs around for a long time, and acts quickly and lethally against insects. DEET is just a repellant, and
I can get that anywhere. I want something to kill the bastards when they decide to rest on my walls -- which is -just- what DDT was designed to do. I'm used to dealing with toxic chemicals, but I'd like to try making some DDT 'cause it's essentially harmless to humans in any sane
dosage, it can be painted on surfaces and hangs around for a long time, and acts quickly and lethally against insects. DEET is just a repellant, and
I can get that anywhere. I want something to kill the bastards when they decide to rest on my walls -- which is -just- what DDT was designed to do.

(ALso, FWIW, DDT used in this manner doesn't pose environmental hazards, since it doesn't move along the food chain, so I wouldn't feel guilty about
that.)
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
I have some DDT, in some bottles of preparations made to kill borers and spiders about 40 or 50 years ago. I am holding on to them, in case they are
ever needed.
|
|
|
XxDaTxX
Hazard to Self
 
Posts: 66
Registered: 12-3-2006
Member Is Offline
Mood: No Mood
|
|
Just so you know. DDT DOES undergo biological magnification. To put it briefly, as it travels up food chains it can effectively concentrate millions
of times stronger then the initial level that it was introduced as. Although this may not mean much to you, it meant alot the the bald eagle
population, whale populations, and other species at higher trophic levels. Do a search. Its does happen. I did a semester's worth of research in a
related field not too long ago.
|
|
|
ziqquratu
Hazard to Others
  
Posts: 385
Registered: 15-11-2002
Member Is Offline
Mood: No Mood
|
|
XxDaTxX, if you actually read Sev's post, you'd never have made your comment. Certainly, DDT DOES undergo biomagnification, leading to severe
problems for a variety of species. However, if you use it carefully, and particularly in an application such as coating walls indoors, then there's
not a problem. It's all about taking necessary precautions to ensure the possibility of negative effects is minimised. Sev, reading his post,
recognises that fact! It can only biomagnified if it's eaten, and then the thing that ate it is eaten, and then... So unless Sev's cat likes the
taste, and and also like to visciously harrass the doberman up the road, there's no problem.
As a side note, DDT is still relatively widely used in areas of the world where malaria is endemic, since the anopheles (sp?) mosquito, which is
responsible for transmitting the disease, is still distinctly sensitive to it (whereas many other species have developed a marked resistance). Since
these areas are generally third world and thus poor, and since DDT is quite cheap, it is the insecticide of choice for trying to control mosquito
populations.
[Edited on 5-6-2006 by ziqquratu]
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
DDT is sufficiently volatile that it will, in spite of ziqquratu's protestations to the contrary, enter the food chain.
Go for one of the pyrethrins- much more bio friendly and you get to grow pretty flowers.
|
|
|
Sev
Harmless

Posts: 5
Registered: 3-6-2006
Member Is Offline
Mood: No Mood
|
|
Thank you, ziqquratu, you summed up exactly my thoughts.
| Quote: | Originally posted by unionised
DDT is sufficiently volatile that it will, in spite of ziqquratu's protestations to the contrary, enter the food chain.
Go for one of the pyrethrins- much more bio friendly and you get to grow pretty flowers. |
First off, I wouldn't be using this on a large scale. I don't think that volatilized DDT from an application on the walls of a few rooms would enter
the food chain in any appreciable way, even considering biomagnification. I'd be making a few grams of it, hucking it in a spray bottle with alcohol,
and spraying the walls down once. The small amount of chemical involved and the huge amount of dilution in air means that the amount entering the
food chain is negligible.
Secondly -- pyrethrins, from what I've read about DDT and seen with them, don't work as well. And they're more toxic to higher life forms, too --
though that's still a nonissue with the small amounts involved.
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
"I wouldn't be using this on a large scale."
Most people didn't; that didn't stop it being a problem.
"Secondly -- pyrethrins, from what I've read about DDT and seen with them, don't work as well. And they're more toxic to higher life forms, too "
They work well enough for most people and, since they biodegrade and aren't stable in sunlight, the toxicity really isn't a problem.
Since you aren't going to get hold of chloral (Or at least, not legally or easily) I don't suppose it matters.
|
|
|
future625
Harmless

Posts: 3
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
Chloral should be a relatively easy preparation. prepare acetaldehyde by one of the methods on the board such as dehydrogenation of ethanol or
dehydration of glycol, add a few drops of muriatic acid and pass chlorine gas into the solution giving mono, di and/or trichloroacetaldehyde.
to get chlorobenzene one opens the catalog dials the 1800 number and reads off his digits. or Mothballs contain p-Dichlorobenzene one could likely
dissolve them in ether and reduce to monochlorobenzene w/ magnesium metal.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2732
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by unionised  | "I wouldn't be using this on a large scale."
"Most people didn't; that didn't stop it being a problem."
"Secondly -- pyrethrins, from what I've read about DDT and seen with them, don't work as well. And they're more toxic to higher life forms, too "
|
DDT was used in ton scale for agricultural use and air spraying for mosquito control, which is where most environmental exposure come from. It would
have never had the issues it did if people were using it is small amounts inside only.
The same for chlordane, which was a valuable chemical for termite treatment, but was banned due to people using it in large fields as a agrochemical
omni-pesticide. When used in termite control, a pound could treat a house for 40+ years, and it sticks to clay very strongly, so it does not leach
out much into water, which many other pesticide do.
The problem with mankind is that frequently once we find something useful, people over use it until it becomes a problem. The same could be said for
antibiotic use in farm animals, and many other chemicals. If people used more common sense (yes, it is an oxymoron), then many of the problems of
mankind would not exist.
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Is the Original Poster still here half a dozen years on.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Ummm....
No!
http://www.sciencemadness.org/talk/member.php?action=viewpro...
|
|
|
Vikascoder
Hazard to Others
  
Posts: 309
Registered: 28-1-2012
Member Is Offline
Mood: No Mood
|
|
Why you are making DDT it will leads to biological magnification
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
http://en.wikipedia.org/wiki/DDT#Effects_on_human_health
It is not benign...
I weep at the sight of flaming acetic anhydride.
|
|
|
Organikum
resurrected
    
Posts: 2337
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
DDT is very effective and comparable nice to the environment if not sprayed onto the fields by the ton.
But for to keep a room free from vermin it is sufficient to mix pepper or chili-powder or plain capsaicin into the paint.
Works since a thousand years and more...
|
|
|
Funkerman23
Hazard to Others
  
Posts: 416
Registered: 4-1-2012
Location: Dixie
Member Is Offline
Mood: No Mood
|
|
Not to derail the thread but did that book come from the library here or is it a scanned section of one of your own books?
That aside DDT while notorious might not be the best compound for the job given. But ehh I am not all knowing..
|
|
|
ripple
Harmless

Posts: 19
Registered: 19-1-2012
Member Is Offline
Mood: No Mood
|
|
I think the term 'biomagnification' is misleading and confusing. I've always heard it the problem being referred to as 'bio-concentration' or
"bio-accumulation"
'Magnification' makes it seem like its effect or quantity is increased through the food chain, when really it is just concentrated.
Practically speaking, the amount that any individual at home could produce could never contribute to the problem of 'bio-accumulation' or
'bio-concentration' on an appreciable scale.
The term is mostly used to describe things that are absorbed and NOT 'bio-degradable', i.e., even at 100% transmission through the food chain, you'd
need to have a pretty isolated environment for 100g of DDT to end up in 1 bird (exaggerated for emphasis). The problem of bioaccumulation is based on
industrial scale use of a chemical and it being ubiquitous in a food source, NOT resulting from a single point source of the material.
No DDT is CREATED by it travelling up the food chain, it is only concentrated in organisms feeding from a uniformly or highly contaminated food
source. It is banned to avoid its ubiquity, not because its effects amplify through the food chain.
|
|
|
carothers
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
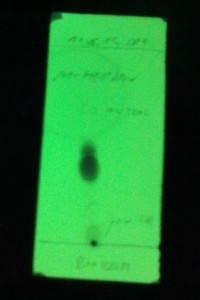
DDT synthesis by the procedure above
4.2 mL (4.662 g, 41.4 mmol) of chlorobenzene and 4.0 g (24.2 mmol) of chloral hydrate were mixed in a 100 mL 1N RBF and heated until chloral hydrate
had dissolved. 36 mL of concentrated sulfuric acid was added slowly and the flask was closed with drying tube. Subsequently, a white turbidity
appeared and this was set to be magnetically stirred over the weekend, however at some point the stir bar got stuck.
White solid spheres were formed over the weekend and the reaction mixture turned brown. TLC of the solid shows two spots (Developed in n-hexane,
detection UV). Reaction mixture was poured into a 400 mL beaker half filled with ice-cold water. A fairly exothermic reaction was observed. After
cooling, water was decanted of the solid and the solid was first washed with 50 mL of 5 % sodium bicarbonate solution then with 3x25 mL of water. This
was vacuum filtered and left to dry on air. Still slightly wet solid was transfered into a clean 100 mL Erlenmeyer flask and dissolved in 25 mL of hot
isopropanol (Used too little!). After cooling, a fine white crystalline solid formed which was then vacuum filtered and dried under vacuum. Yield 3.23
g (44.0 %) of white powder that has a slight apple odor, mp 100-103 °C.
Theoretical mass is 7.34 g (20.7 mmol) and the literature mp is 108.5 °C.
TLC of the final product shows traces of the second spot, presumably o,p isomer. 1H NMR shows the o,p isomer as impurity.
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Nice write-up, and welcome to the forum!
|
|
|
carothers
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
Thanks!
It would be interesting to test the usefulness of this material, especially where the exposure to it would be minimal. One particular example comes in
mind - wood boring insects. A solution of DDT could be made and injected into exit holes which this pest make, killing them (presumably) for years and
years later. DDT should be superior to commercial pyrethroids or organophospates even if it is half as effective because it degrades much slower and
is not toxic as organophospates are.
|
|
|
HeYBrO
Hazard to Others
  
Posts: 289
Registered: 6-12-2013
Location: 'straya
Member Is Offline
Mood: 
|
|
Yes, however DDT has huge environmental impacts, so i would not suggest doing so...
|
|
|
foxofax474
Harmless

Posts: 11
Registered: 17-4-2023
Location: USA
Member Is Offline
Mood: Still processing
|
|
Quote: Originally posted by carothers  | | TLC of the final product shows traces of the second spot, presumably o,p isomer. 1H NMR shows the o,p isomer as impurity.
|
Is there a way to purify the product to get rid of the o,p isomer or any DDE/DDD in there?
I checked pubchem and a recrystalization might be possible?, maybe in like benzene or something
| Quote: | | Practically insoluble in dilute acids, alkalies. ... Freely soluble in pyridine, dioxane; solubility in organic solvents increases with rise in
temperatures. |
| Quote: | | Solubility, g/100 mL solvent: 58 acetone; 78 benzene; 42 benzyl benzoate; 45 carbon tetrachloride; 74 chlorobenzene; 116 cyclohexanone; 2 g/100 ml 95%
alcohol; 28 ethyl ether; 10 gasoline; 3 isopropanol; 8-10 kerosene; 75 morpholine; 11 peanut oil; 10-16 pine oil; 61 tetralin; 50 tributyl phosphate.
|
But this still makes me uncertain if the impurities would recrystallize out with it (especially the o,p isomer)...
Of course i'd imagine doing a column would work but thats pricey and quite involved lol so idk
(∩^o^)⊃━☆ the proof is by magic
|
|
|
| Pages:
1
2 |