| Pages:
1
..
5
6
7
8 |
woelen
Super Administrator
        
Posts: 8090
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
A picture of the dried powder in a watch glass. The material has a beautiful bright color, it really looks like it is in the picture with a deeply
saturated color.

|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Woelen, did you ever get round to making any violurate salts?
Since you violuric acid is strongly pink, whereas the acid I received from Rusty is creme coloured, I'm all the more curious about your results.
|
|
|
woelen
Super Administrator
        
Posts: 8090
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I did not make the free acid, but the sodium salt. I made the free acid only in solution. Such solutions are colorless.
Finding time for making other salts is hard for me now. I am building a large garden house in our garden and that takes nearly all spare time at the
moment. Before winter arrives it must be complete, including all streetwork around it.
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 202
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
Hello everyone! Im back with another few violurates to add to this growing collection.
Thanks to Ormarion for making and sending me pictures of dimethylaniline, 2,2 bipyridine violurates and the zirconium salt. Ormarion has produced
their own violuric acid from diethylmalonate, and is working on a video about the process, although it will be in french, i think video documentation
will be great for those looking to attempt the reactions.
And thanks to h0lx for making and sending pictures of 2-amino-4,6-dimethylpyrimidine and 3,5-dimethyl pyrazole salts.
Myself i havent had time to do more than triisopropanolamine since ive been busy with exams. But i do have some exciting news, thanks to an extremely
generous offer 13C, 1HNMR and FTIR analysis will be done on the violuric acid i have produced aswell as possibly some high end microscope pictures of
a few of the salts. Its possible that even COSY and HSQC scans will be performed. I will update with results when i get them 
The new organic salts
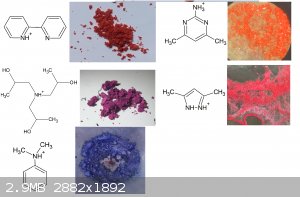
Zirconium salt

[Edited on 31-8-2021 by RustyShackleford]
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Nice work!
I performed violuric acid preparation from alloxan hydrate and hydroxylamine HCl yesterday, but it appears that my alloxan sample is quite degraded,
and reaction did not yield any acid crystals, but the yellow solution turns pink-ish when sodium hydroxide is added.
I will try to purify alloxan hydrate and try again in upcoming days.
|
|
|
Ormarion
Hazard to Self
 
Posts: 59
Registered: 19-12-2017
Location: France
Member Is Offline
Mood: Alkylating her DNA
|
|
Just a little correction, this isn't dimethylaniline violurate wich is blue, it is piperidine  but thanks for sharing my pictures but thanks for sharing my pictures
|
|
|
h0lx
Hazard to Self
 
Posts: 69
Registered: 31-12-2005
Member Is Offline
|
|
All the violurates I made sans the aminoguanidine which I fogot to add to the pic, that I managed to make with the 3g OP sent me. Will continue the
exploratioin of those compounds soon
from left:
ortho toluidine
triethylamine
methylamine
guanidine
3,5-dimethylpyrazole
2-amino-4,6-dimethylpyrimidine
pyridine
triethanolamine
ethanolamine
ammonia
ethylenediamine

|
|
|
RustyShackleford
Hazard to Others
  
Posts: 202
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
Im back with analysis results from 1H, 13C and FTIR 
orange = sample, blue = bruker spectra. a respectable fit i think, good enough to confirm the compound
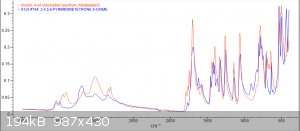
Zoom-ins
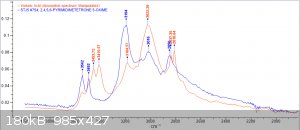

1H performed in DMSO-d6, OH peak probably gone due to water in solvent.
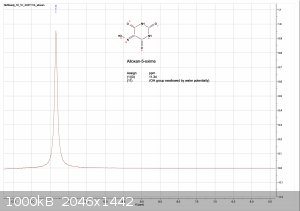
evidence of water in the solvent, the DMSO turned blue at the bottom.

13C. was not soluble enough to provide a good sample solution with chloroform nor DMSO.
|
|
|
Ormarion
Hazard to Self
 
Posts: 59
Registered: 19-12-2017
Location: France
Member Is Offline
Mood: Alkylating her DNA
|
|
Last violurate made
Pink one is B rhodamine
Blue is tributylamine
Red/brown is haematoporphyrin (powder form look deep green)
I am currently making a video on barbituric and violuric acis synth
  
[Edited on 1-5-2022 by Ormarion]
|
|
|
SuperOxide
National Hazard
   
Posts: 539
Registered: 24-7-2019
Location: Devils Anus
Member Is Offline
|
|
Quote: Originally posted by Ormarion  | Last violurate made
Pink one is B rhodamine
Blue is tributylamine
Red/brown is haematoporphyrin (powder form look deep green)
I am currently making a video on barbituric and violuric acis synth |
Those are great! I plan on making some of the same products eventually. They look too amazing to not try.
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Quote: Originally posted by Ormarion  | Last violurate made
Pink one is B rhodamine
Blue is tributylamine
Red/brown is haematoporphyrin (powder form look deep green)
I am currently making a video on barbituric and violuric acis synth
[Edited on 1-5-2022 by Ormarion] |
I'm looking forward to seeing that video
|
|
|
kmno4
International Hazard
    
Posts: 1517
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Hm....
There is very old reference (see "Uric acid" at Wikipedia), describing uric acid formation from glycine and urea*.
If it really works, then it makes preparation of VA very cheap, even if total yield of this multistep synthesis is low. I have ordered some larger
amount of glycine to test this UA preparation, just for fun.
But maybe someone here already tried this ?
* it is hard to imagine exact machanism of this high temperature reaction, but it seems that it has something to do with Strecker degradation.
ps. playing with guano is not my cup of tea
[Edited on 13-5-2022 by kmno4]
Слава Україні !
Героям слава !
|
|
|
Boffis
International Hazard
    
Posts: 1903
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Woow its a while since I posted that.
Like you say if you can make uric like the paper describes it would be excellent even if not very efficient. The original paper doesn't state the
yield.
The di-potassium and di-sodium salts are fairly soluble in a small excess of the same caustic alkali and then the free acid ppt'd with dilute HCl or
sulphuric acid. The mono-sodium salt can be ppt'd by sodium bicarbonate. So it may be possible to avoid the precipitation with silver and the H2S
treatment. The solubility of free uric acid is very low unless certain organic bases are present. Dont overheat the solution or heat for long periods,
this decomposes the uric acid.
For those that don't have access to the paper or can't read German I have attached a quick translation.
@kmno4; What's wrong with the Peruvian bird guano? The stuff sold as an organic fertilizer is sterilized and the uric acid concentration is >20%.
Attachment: Preparation of Uric acid Monatshefte Horboczawski 1882 English.docx (14kB)
This file has been downloaded 315 times
|
|
|
kmno4
International Hazard
    
Posts: 1517
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
I have received the parcel, full of chemical goods 
I do not trust mentioned paper very much, it looks more like patent, but not scientific publication. I could not find any more paper with confirmation
of the procedure by Horbaczewski.
It is the simplest one, among the others, much more complicated methods of UA preparations - and no-one exploits it ....
But I have now 250 g of glycine to waste, we will see
I am going to use hot air gun, instead of ancient metal bath.
ps_1. this extremelly low solubility of UA, compound with very polar molecules, still surprises me, hah.
ps_2. preparation of barbituric acid from malonic acid, urea and Ac2O in AcOH is in my reach at once but I want to try UA method, just for
(scientific) fun.
[Edited on 16-5-2022 by kmno4]
Слава Україні !
Героям слава !
|
|
|
Boffis
International Hazard
    
Posts: 1903
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi kmno4, The paper is very old and is very much on a par for the period I would say, having looked at many old papers from this period. I am quite
sure the results will be reproducible, the problem is that it may not be uric that is produced, he also doesn't give a yield either! I suspect that
isocyanuric acid is also produced along with diketopiperazines and, in order to produce uric acid, a lot of ammonia would need to be expelled so
guanidine is another possible byproduct. The isocyanuric acid may be difficult to remove from the uric acid. I would try it myself but I am currently
away from home.
The low solubility of uric acid is almost certainly due to the many potential hydrogen bonding sites on the molecule.
Please let us know how you get on.
|
|
|
Ormarion
Hazard to Self
 
Posts: 59
Registered: 19-12-2017
Location: France
Member Is Offline
Mood: Alkylating her DNA
|
|
And there you go, new video on barbituric and violuric acid synth, if anyone speak french here feel free to add subtitles i don't really have time
from my side
https://www.youtube.com/watch?v=TN8tew9xg6A
|
|
|
LeLaborantin
Harmless

Posts: 6
Registered: 25-1-2021
Location: Belgique
Member Is Offline
|
|
Un grand merci pour ta vidéo que je viens de regarder !
Continue a en faire plein ^^
|
|
|
kmno4
International Hazard
    
Posts: 1517
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Unfortunately - nothing interesting to report.
Start: urea 50g, glycine 5g
Hot air gun heating works perfecly, the rection goes as described in the paper: melting, a lot of gases, the mixture slowly turns yellow, orange and
finally deeply red, at some instant it becomes turbid and heting was stopped. The post-reaction mixture becomes bright yellow brown and is semi-solid
even below 100 C.
After cooling, urea, biuret etc... was washed out with water, keeping pH ~ 3. Finally, about 9g of bright brown, not crystalline, powder was obtained.
It surely a mixture, expected amount of UA is no more than 5 g. Unfortunaly, further attempts to purify is somehow gave nothig. I ended up with ~5 g
of the same looking powder. It is soluble in KOH solution, giving deeply yellow, practically red solution. When acid is added, a lot of semi-jelly
yellowish-grown precipitade is formed.
It does not look as UA, pity.... Besides, if cyanuric acid is present, it would be hard to separate out.
I am going to try to "do something" with it, but I doubt if UA is formed in this reaction at all.
Слава Україні !
Героям слава !
|
|
|
kmno4
International Hazard
    
Posts: 1517
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Final test, to prove (or not) this bull*t and lose no more time for it.
Oxidation to alloxan by KClO3/HCl, according to reference no.3 given here :
http://www.orgsyn.org/demo.aspx?prep=cv3p0037
About 5 g of prepared powder was taken to reaction. Effects are going to be reported very soon 
.....................
Effects: no alloxan is formed. So, good bye UA from glycine and urea - without regret 
[Edited on 22-5-2022 by kmno4]
Слава Україні !
Героям слава !
|
|
|
Cakethegrate
Harmless

Posts: 4
Registered: 8-12-2022
Member Is Offline
|
|
Violuric acid salts (fantastic colors and variety)
Hey everyone, I'm new here, so apologies if I mess up this post.
I have been getting deep into violurates lately and would like to talk about a few things I've done with them, starting with a mostly otc synthesis to
a derivative of violuric acid which makes colors at least as vibrant as the normal violuric acid, if not better in some cases, it is called
1,3-dimethylvioluric acid and I was able to make it with semi-decent yields in a few steps starting with caffeine. Which I will outline the procedure
for below.
All that is happening in the reaction is an oxidative cleavage of caffeine to yield 1,3-DimethylAlloxan and Monomethylurea, however the desired
product is the dimethylalloxan. Because this reaction is a little messy we need to isolate our dimethylalloxan by reducing it to generate its
insoluble dimer, tetramethylalloxantin which will precipitate out of solution. The tetramethylalloxantin is then treated with RFNA to isolate the
dimethylalloxan again which will crystallize out and can be treated with hydroxylamine which will install an oxime group on the position 5 of the
ketone, which will yield the 1,3-dimethylvioluric acid.
I followed this procedure from illumina to obtain the tetramethylalloxan needed with just a few modifications: https://illumina-chemie.de/viewtopic.php?t=5375
Caffeine to Tetramethylalloxantin:
25g of caffeine is placed into a 2-neck 500ml round bottom flask with a condenser. With stirring on 35ml of conc HCl and 75ml of water is added to the
caffeine until it is dissolved. The flask is warmed in a water bath kept at 50-55C and 10g of potassium chlorate is added in small portions over a
period of 1.5-3 hours, patience is key for better yields and it is important to add the chlorate slowly with small additions, if you add too much at
one time or add it too quickly it will overoxidize your product. Upon addition of the KClO3 the solution will turn yellow and a thick
precipitate of 8-Chlorocaffeine will form which may make stirring difficult, it is reccommended to have a way to manually stir the solution if your
stir bar becomes stuck. Small amounts of presumably Cl2 and ClO2 are formed, however the amount of the gases formed were very
small for me and weren't too concerning. You can know when to add more chlorate based on how yellow your solution is, after a certain interval of time
between additions of chlorate the solution becomes a more pale yellow which is when you can add more KClO3 in small increments. After most
of the KClO3 is added the precipitate of 8-chlorocaffeine will dissolve, at the end of addition the solution is left to stir for 15-30 more
minutes and air is blown through the warm solution for another 30 minutes to remove excess chlorine in solution. The reaction mixture is then cooled
in an ice bath and the reflux condenser is exchanged for a dropping funnel with a solution of 14.1g SnCl2 in 20ml of 18% HCl, which is
slowly dripped into the solution. After complete addition a white powder of tetramethylalloxantin begins to precipitate out. To increase the amount of
precipitate 5ml of 35% H2O2 is added and the solution is stirred for another 30 minutes on the ice bath. The precipitate is then
suction filtered to yield 12.4g of tetramethylalloxantin.
1,3-DimethylAlloxan monohydrate from TetramethylAlloxantin:
This reaction is surprisingly very clean and doesnt really require measuring in exact amounts, however you can also follow the procedure from orgsyn
that kmno4 already posted: http://www.orgsyn.org/demo.aspx?prep=cv3p0037
I usually just measure out the desired amount of TetramethylAlloxantin, wet it slightly with distilled water, put it on a hotplate with stirring and
heat the solution so it is slightly warm to the touch, then slowly pipette drops of fuming nitric acid. NO2 will evolve and the
TetramethylAlloxantin quickly dissolves. Once all the TetramethylAlloxantin is dissolved and no more NO2 is generated the solution is taken
off the hotplate and is cooled for over 10 hours. 1,3-DimethylAlloxan monohydrate crystallizes out during this time in large, clear/colorless crystals
as seen here.

1,3-DimethylVioluric Acid monohydrate from 1,3-DimethylAlloxan monohydrate:
2g(10.63 mmol) of 1,3-DimethylAlloxan is dissolved in 3-4ml of distilled water and 0.87g(12.5mmol) of Hydroxylamine HCl is added with heating and
stirring. After ~5-10 minutes a large amount of 1,3-DimethylVioluric acid crashes out. The reaction mixture was then cooled and filtered off to yield
2.241g of white/cream colored 1,3-DimethylVioluric Acid monohydrate.
To make the salts the 1,3-DimethylVioluric Acid is dissolved in ethanol and an equimolar amount of the base is added, the salt will typically
immediately precipitate upon addition of the base.
To prove that I have made the 1,3-DimethylVioluric Acid I made the Potassium salt which can be seen below.

Anyways, this post is getting a little long, so I should probably end it here, but as a side note I have also made about 12 amino-acid violurates,
most of which haven't been made before, so if you would like to see another post about those let me know.
[Edited on 2-3-2023 by Cakethegrate]
|
|
|
j_sum1
Administrator
       
Posts: 6377
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Love to see the pix.
Attach the file as an attachment: the button can be found when you click "edit post" or "preview post".
And yes, we would love to see the amino acid acid salts.
[Edited on 2-3-2023 by j_sum1]
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 202
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
Absolutely post the pictures of all the stuff youve made!
Being able to create Violuric acid (/dimethyl) from a much more accessible starting material is tremendous! especially for the Americans out there for
whom I've heard barbituric acid is quite difficult to get.
|
|
|
Cakethegrate
Harmless

Posts: 4
Registered: 8-12-2022
Member Is Offline
|
|
Hey everyone, I'm back with more pictures  . This time I will be uploading
photos of the amino acid violurates and 1,3-dimethylviolurates(most of which are new), along with the basic procedure I did to make most of them. . This time I will be uploading
photos of the amino acid violurates and 1,3-dimethylviolurates(most of which are new), along with the basic procedure I did to make most of them.
Procedure from violuric acid monohydrate:
.5g(2.86mmol) violuric acid monohydrate is dissolved in 50ml of warm EtOH. To this solution, an equimolar amount of amino acid/amine is dissolved in a
minimal amount of water then added to the solution of violuric acid monohydrate, many of the organoammonium salts precipitate immediately, which can
then be filtered off, however if it is soluble it will typically be boiled down to around half the original volume then cooled until the salt is
completely precipitated.
The reason I use ethanol as a solvent instead of water is because of a few reasons. The first is of course that the solution will evaporate down
easier and quicker without as much risk of the organoammonium salt decomposing(which is much easier to do in water, many organoammonium violurates
decompose at around 75C). The second reason is because the solubilities are much better to deal with in ethanol. In water most organoammonium
violurates are very soluble and often require being boiled down to near dryness to obtain the salt whereas with ethanol that is almost never the case
and typically the organoammonium salts will precipitate in ethanol with relative ease.
Anyways, here are the amino-acid violurates as promised. (From left to right) Is Alanine Violurate, Arginine Violurate, Asparagine Violurate, Aspartic
Acid Violurate, Histidine Violurate, Isoleucine Violurate, Lysine Violurate, Methionine Violurate, Phenylalanine Violurate, Proline violurate,
Threonine Violurate, and Valine Violurate.
I especially like the colors of the Lysine, Phenylalanine, Valine, Histidine, and Arginine salts. If anyone wants any of the violurates absorbance
spectra(in water) I can provide that upon request.
           
Here are a few amino acid salts made with the 1,3-Dimethylvioluric acid, its interesting how different the same amino acid violurates and
dimethylviolurates can be, those two methyl groups seem to make a surprising difference. so far I have noticed that the amino acid dimethylviolurates
tend to be more shifted towards the blue/green end of the spectrum rather than the red/pinkish that seems typical for the normal amino acid
violurates. I'm planning on making the dimethylammonium
1,3-dimethylviolurate salt(I know, a lot of methyl in the name lol) however, I haven't gotten around to it yet.
In order from left to right is Proline 1,3-Dimethylviolurate, Phenylalanine 1,3-Dimethylviolurate, Niacin 1,3-Dimethylviolurate, Tryptophan
1,3-Dimethylviolurate, and Leucine 1,3-Dimethylviolurate.
   

Also, if anyone happens to have some theobromine lying around, you can use it to make methylvioluric acid (monomethyl derivative of violuric acid)
using essentially the same procedure as I described in my last post to make 1,3-dimethylvioluric acid from caffeine, as shown in the paper below,
sorry it's in German.
Attachment: biltz1912.pdf (819kB)
This file has been downloaded 199 times

[Edited on 6-3-2023 by Cakethegrate]
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Thanks for the pictures, beautiful.
|
|
|
Ormarion
Hazard to Self
 
Posts: 59
Registered: 19-12-2017
Location: France
Member Is Offline
Mood: Alkylating her DNA
|
|
Hey everyone hope you doing alright. I recently wanted to share some of the pics of thiovioluric acid i made after talking about it with Cakethegrate,
i used exactly the same procedure as i made a video about to make the barbituric acid but replacing urea with thiourea. Synthesis seemed faster too as
well as for the thiovioluric acid (it is also way less soluble in water than violuric acid).
To make those thioviolurate i dissolved the acid in warm acetone and slowly added the base , and then let it stirred for a bit.
I recommend you do not heat them as when i tried for some they decomposed releasing stinky sulfur smell.
Im not rly aware about how to share pics on post already made and as there is quite a lot of pics here is a little google drive link to look at them,
enjoy and feel free to ask anything :3
(if im braveenough i might try making the selenium and tellurium ones)
https://drive.google.com/drive/folders/1gM-DAlawAKPvivxLLaQt...
|
|
|
| Pages:
1
..
5
6
7
8 |