| Pages:
1
..
3
4
5 |
myristicinaldehyde
Hazard to Others
  
Posts: 166
Registered: 23-4-2016
Location: .͐͌ ͛҉̻̫̰̻̖E̮ͮ̐́̚ ̢̗̅̉ͩ͂̒̌.̯̻̺̯̀̎͂̄ͩ̚
Member Is Offline
Mood: сорок пять
|
|
The simplest answer is that LAH is used strictly in anhydrous conditions, and reacts aggressively with any water that would form. Hydrolysis requires
water. Ergo, LAH does not produce the hydrolyzed product.
If we don't study the mistakes of the future we're doomed to repeat them for the first time.
|
|
|
sulfuric acid is the king
Hazard to Self
 
Posts: 94
Registered: 11-1-2017
Member Is Offline
|
|
What are differences of both methodes?I am still learning,i am interested in details...
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
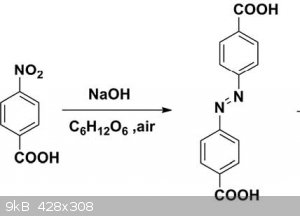
Below is a procedure for making an azobenzene that I am not familar with. Can anyone give me a name for the reaction or describe the mechanism.
Procedure, 4-nitrobenzoic acid (2.0 g, 12 mmol) and NaOH (5.75 g, 144 mmol) were mixed in 30 mL of water and heated until the solid dissolved. Glucose
(13.0 g, 72 mmol) aqueous solution (20 mL) was added drop wise at 70C, initially yielding a yellow precipitate and subsequently a brown solution upon
further addition of glucose. A stream of air was bubbled into the mixture overnight, resulting in a light brown precipitate, and then the precipitate
was filtered. The cake was dissolved in 10 mL of water, and acetic acid was used to adjust the pH of the solution in the range of 5 to 6.
Approximately 1.06g azobenzene-4,4 dicarboxylic acid as light pink powder was obtained by filtration, washed with 25 mL of distilled water, and dried
to constant weight in an oven at 80C
I found this note Attachment: aaaaglurednitro.pdf (286kB)
This file has been downloaded 1121 times
|
|
|
TGSpecialist1
Hazard to Self
 
Posts: 53
Registered: 24-12-2017
Member Is Offline
Mood: always tired
|
|
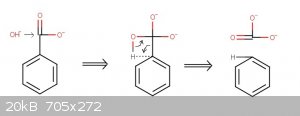
What is the mechanism of decarboxylation by molten NaOH/KOH?
For benzoate I think it's like in picture, but I'm not sure how it works for saturated carboxylate salts.
|
|
|
TGSpecialist1
Hazard to Self
 
Posts: 53
Registered: 24-12-2017
Member Is Offline
Mood: always tired
|
|
re:sulfuric acid is king
I'm sure that your nitroalkene can be directly be reduced to sec-butylamine with aluminium amalgam, since P2P + nitromethane + Al(Hg) reaction works
to produce methamphetamine: https://erowid.org/archive/rhodium/chemistry/ritter-alhg.htm...
Nitroalkenes are prone to hydrolysis so the reaction has to be done in methanol or the product will be contaminated with 3-aminobutan-2-ol.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
How do you use the Sandmeyer reaction to create a phenol in acidic solution?
|
|
|
CharlieA
National Hazard
   
Posts: 646
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
My guess: starting with -NO2 or -NH2 prepare the diazonium salt with NaNO2/HCl and hydrolyze to the phenol.
My reference: Janice Smith, Organic Chemistry (2nd edn.), page 985.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
The question is how to hydrolyze to a phenol in an acidic solution. Copper (I) hydroxide would be immediately destroyed, and while water is said to
function to convert diazonium salts to hydroxides without copper catalysis, it's not clear how that works.
Edit: Apparently it works by just heating the diazonium salt in water. From what I gather, the reaction is SN1 with the rate determining step being
the formation of the carbocation when the diazonium group leaves. I guess the carbocation attacks a water molecule and deprotonates it... then the
proton meets up with the diazonium ion and generates acid and gaseous nitrogen?
So basically just crank up the temperature slowly until gas starts coming off and keep it at that temperature until the gas stops? (Oh and also
probably don't do this in 60% sulfuric acid even though it's SN1, right?)
[Edited on 3-9-2018 by JJay]
|
|
|
j_sum1
|
Thread Untopped
1-12-2018 at 16:28 |
j_sum1
|
Thread Topped
1-12-2018 at 16:29 |
MUSSUM
Harmless

Posts: 3
Registered: 24-4-2019
Member Is Offline
|
|
Hey man look at this book.
Pushing electrons, this book explain the electron flow, so very simple form, as simple as Kumon math course.
You make the download in this link:
https://b-ok.cc/book/2074519/c5f5b6
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
"Many reactions are very well established by experimental methods. I don't know how familiar you are with labelling studies, kinetic isotope effects,
ultrafast spectroscopy, etc."
Thank you!
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
| Pages:
1
..
3
4
5 |