| Pages:
1
..
95
96
97
98
99
..
104 |
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Thank you for the time you've taken to reply, yes i appreciate your insight and would welcome the references you mentioned. Both of my inquiries
you've taken time to assist me, your contribution is very positive and well received........solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
AvBaeyer
National Hazard
   
Posts: 651
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Solo,
Pleased to know you appreciate my comments. I must confess, however, that I was enjoying my after-dinner glass of port when I wrote my comments. I
mentioned the nitration of 1-phenylethanol as a possible starting point which could be nitrated. I do not think that alcohol would survive nitration
even if protected. I think starting from acetophenone would be a much better starting point. So much for that.
As for references, here is what I have and with one exception they are all patents.
Non-patent reference: J. Keck et al., Arzneim-Forsch., (1972), v22, 861 and 869 (two articles, synthesis and pharmacology)
For Synthesis 1 (per my previous post):
DOS 1,793,419 (Thomae)
BE 704,213 (Thomae)
US 3,536,712 (Boehringer Ing)
For Synthesis 2:
DOS 2,157,040 (Thomae)
DE 1,543,928 (Thomae)
Additional Patents:
DAS 2,354,959 (Thomae) - alternative synthesis
US 3.536,712 (synthesis)
DOS and DAS are German patents, that is all I can tell you.
My information comes from:
Dictionary of Pharmacological Agents
A. Kleeman, et al., "Pharmaceutical Substances, Syntheses and Patents," 4th ed, 2001 (Georg Thieme Verlag)
Merck Index
Happy reading! Now to my evening port.
AvB
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
It came to mind that if i can manage to oxidice ethy acetate via some alkane intermediate , i can synthesise acetic anhydride.....any tips on how
can i go about accomlishing this.......solo
ethyl acetate
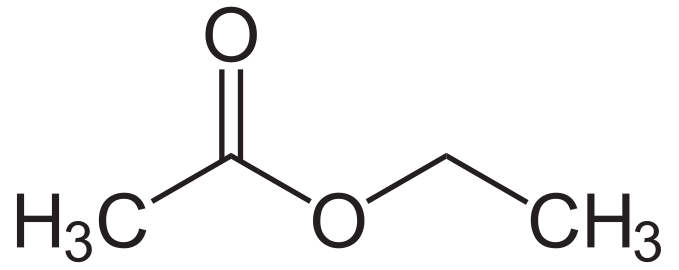
acetic anhydride
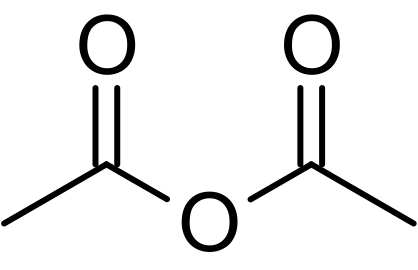
Note: however i did read this "Alkanes usually resist oxidation with oxidising agents such as KMnO4, K2Cr2O7 etc. However, alkanes containing tertiary
hydrogen can be oxidised to corresponding alcohols."
Note 2:how abot a transterification with totsyl chloride hecemaking it an acidic transterification adding sodium acstate to replace the totsyl
chloride all sone in toluene.....just a thought
[Edited on 2-3-2022 by solo]
[Edited on 3-3-2022 by solo]
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2799
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
If you have tosyl chloride, you can just make acetic anhydride from that by reacting with sodium acetate. Boom.
The problem with oxidizing ethyl acetate is that it will tend to oxidize at the acetyl carbon rather than the carbon you want to oxidize, giving
instead ethyl glycolate or ethyl glyoxylate. Maybe if you tried to do a radical halogenation of benzyl acetate, instead, you could get acetic
anhydride or acetyl chloride upon rearrangement. In fact, that might even be a good idea.
Attachment: he2011.pdf (866kB)
This file has been downloaded 376 times
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
I tried to grow some rochelle's salt crystals... it seemed pretty foolproof: neutralize potassium hydrogen tartrate with sodium carbonate until
effervescence ceases, then let it slowly evaporate. Mine has become a thick syrup that refuses to solidify... has anyone encountered this?
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Quote: Originally posted by clearly_not_atara  | If you have tosyl chloride, you can just make acetic anhydride from that by reacting with sodium acetate. Boom.
The problem with oxidizing ethyl acetate is that it will tend to oxidize at the acetyl carbon rather than the carbon you want to oxidize, giving
instead ethyl glycolate or ethyl glyoxylate. Maybe if you tried to do a radical halogenation of benzyl acetate, instead, you could get acetic
anhydride or acetyl chloride upon rearrangement. In fact, that might even be a good idea. |
thaks for that, however a bit puzzled by this.....
"As swim said,I mean Dry distillation.(i did this method many times with Tosyl chloride and even Benzene sulfonyl chloride)
Try 2:1 and 3:1 ratio of sodium acetate : Aryl Sulfonyl chloride"
[Edited on 11-3-2022 by Waffles SS]
,,,,source Acetic Anhydride preparation thread
[Edited on 15-3-2022 by solo]
[Edited on 15-3-2022 by solo]
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
.......well after doing the reaction with the ratios 3:1 , sodium acetate: toluene sulfonyl choride...using 30 grams sodium acetate : 10 grams of
toluenesulfonyl chloride , i got 15 ml of a liquid, still not analysed or redistilled ther was no bang!!!, however i did place my reaction vessel in a
metal cage ....just in case.....solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
…… result, not promising…. I will do the reaction again and will do the reaction on a sand medium keeping temp at 80C …. Keeping reactants
from carbonizing …..solo
note : Waffles can you give any other details on your procedure as i seem i didn't have any success....thanks

[Edited on 23-3-2022 by solo]
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
artemov
Hazard to Others
  
Posts: 181
Registered: 22-8-2018
Member Is Offline
|
|
Can isobutyl nitrite be used in place of n-butyl nitrite for azide and dimethylglyoxime synthesis (I only have isobutanol)?
I keep reading about n-butyl nitrite in various synthesis, rarely other butyl nitrites ...
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Will plain lithium metal be able to react with phenylacetylene to form lithium phenylacetylide?
I know that one would generally use some sort of a strong base like lithium hydride or amide etc...
But what about the metal? Will it be strong enough to replace the hydrogen? If not, then why?
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
goal is to make 4-amino acetophenone
theory....use ethyl p-aminobenzoate (benzocaine) and ethylacetate in a cleisen condensation in ethanol with Naoetoh , because its a base driven
reaction the amine group on the benzene ring won't be affected....however the reaction requires Hcl in order to keep the reaction to the right, now
since i want to decarboxylate the alpha-beta ketone that requires an acid and heat.
now all this acid use concerns me since the amine on the benzene ring can be affected , any thoughts of an alterantive or is my premise wrong and i
could just heat the solution right after the alpha beta ketone is formed and avoid the use of an acid( whichi doubt) ....solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
.....actually there was no need to be concerned with the addition of acid, and the possible reaction with the benzyl amine, after the claisen
condensation reaction in order for the reaction to remain to the right.....solo
[Edited on 12-5-2022 by solo]
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by mackolol  | Will plain lithium metal be able to react with phenylacetylene to form lithium phenylacetylide?
I know that one would generally use some sort of a strong base like lithium hydride or amide etc...
But what about the metal? Will it be strong enough to replace the hydrogen? If not, then why? |
I don’t
think it would work. Normally using a base like lithium amide or an alkyl lithium, it’s essentially just a simple acid-base reaction. You have a
strongly basic anion that deprotonates the alkyne. Li+ as a spectator. With lithium metal, now there’s a redox reaction that needs to occur. Li
needs to get oxidized from 0 to +1. Normally this is done by reacting the metal with alkyl halides. Good electrophiles. The metal reduces both the
carbon and the halide so 2 Li + RX -> RLi + LiX. In your case, you don’t have a good electrophilic, you’re asking it to form lithium hydride as
a byproduct instead. Doesn’t sound so promising. My recommendation, use NBS to convert the phenylacetylene to (bromoethynyl)benzene (I’ve done
this reaction before- be warned, the product smells awful) and react that with lithium metal to get your lithium phenylacetalide plus LiBr.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
I want to chlorinate 4-amino acetophenone not sure if i have to use a metal as a catalyst FeCl3 with a chlorinaing agent.....i saw a reaction where
they used TCCA in MeCN at RT for chlorination ....confused .....any suggestions as to chlorinating agent and solvent with or without a metal
catalyst.....solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
AvBaeyer
National Hazard
   
Posts: 651
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Solo,
Solo:
4-Aminoacetophenone has been iodinated with KI/hydrogen peroxide.
Reddy et al., Synth. Commun. 38, 3894 (2008)
2-Aminoacetophenone has been brominated with KBr/sodium perborate i acetic acid
Roche et al., Tetrahedron Letters 41, 2083 (2000)
o- and p-aminobenzoate esters have also been halogenated similarly to the above but I cannot find the exact reference.
You should be able to accomplish chlorination of your compound with KCl/Oxone in water-methanol or water-acetonitrile.
do a Google Scholar search on papers by N.Narender using the terms "oxyiodination", "oxybromination" and "oxychlorination"to get leading references.
AvB
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
.....thank you for your support and advice, slowly i'm working myself to the complete synthesis of clenbuterol......solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
AvBaeyer
National Hazard
   
Posts: 651
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Solo,
Here is the bromination of p-aminoacetophenone with NaBr/Oxone in aqueous acetonitrile. I expect that chlorination should also work with NaCl or KCl.
Lee, et al., Bull. Korean Chem. Soc. 23, 773 (2002).
AvB
[Edited on 14-5-2022 by AvBaeyer]
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Does a Claisen cross condensation need to be an anhydrous reaction? I seem to be getting my ester hydrolysed and no beta keto ethyl ester.....I've
run the reaction 5 times with the same results even though i changed the addition of reagents in different order ....Hence a friend suggested i use
anhydrous ethanol dry my esters....k.
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
AvBaeyer
National Hazard
   
Posts: 651
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
Dry, very dry.
AvB
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
......after distilling all of my solvents since i didn't have and anhydrous, the cleisen cross condensation finally worked for me after 7 attempts
.....therefore 4-amino acetophenone with a MP of 105-106˚C was acquired with broken glass clear crystals ....thanks for the assistance......as the
chlorination is next.......solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
....i have terbutyl alcohol and need to make terbutyl amine, thought of first making terbutyl chloride with HCl then treating it with aqueous
ammonia......any thoughts would be appreciated.....solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by solo  | | ....i have terbutyl alcohol and need to make terbutyl amine, thought of first making terbutyl chloride with HCl then treating it with aqueous
ammonia......any thoughts would be appreciated.....solo |
That will probably work, although the presence of water might muck it up. It's an Sn1 reaction- the carbocation will form, and then react with
whatever is closest. You might want to dissolve the ammonia in a non-nucleophilic solvent and then add the t-butyl chloride.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
AvBaeyer
National Hazard
   
Posts: 651
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
The reaction of t-butyl chloride with ammonia is destined for failure. t-Butyl chloride is a tertiary halide and will undergo elimination to
isobutylene in the presence of ammonia more rapidly than any other reaction.
You can react your t-butanol with acetonitrile in a Ritter reaction to get N-t-butylacetamide. However, I would not bet on an easy hydrolysis of this
compound to get t-butylamine. You will need to look up the hydrolysis conditions for such highly hindered amides.
AvB
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2799
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Might consider a Reformatsky reaction of chloroacetate ester and acetone, which eliminates to a 3,3-dimethylacrylate that can, as I understand it,
attack ammonia as an electrophile "properly".
Of course, this route is totally different.
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
......I saw this,....solo
Thermodynamic study of direct amination of isobutylene to tert-butylamine
Shangyao Gao
Chinese Journal of Catalysis
38 (2017) 106–114
Abstract
On basis of thermodynamic empirical equations, the thermodynamic parameters for the direct
amination of isobutylene to tert‐butylamine, an atomically economic and green chemical reaction,
were calculated. In particular, the equilibrium conversion of isobutylene under various reaction
conditions close to those used in industry was calculated and discussed. Isobutylene amination is a
temperature sensitive reaction due to its exothermic nature and isobutylene equilibrium conversion
decreases with temperature. However, kinetically, the amination reaction will be faster at a
higher temperature. Thus, there must be an optimum temperature for the reaction. A high pressure
and n(NH3)/n(i‐C4H8) molar ratio promote the transformation of isobutylene to tert‐butylamine.
Developing a highly efficient catalyst under mild reaction conditions is preferred for the amination
process. The reaction was investigated over a series of acidic zeolites. ZSM‐11 zeolite exhibited the
best performance with 14.2% isobutylene conversion (52.2% of the equilibrium conversion) and >
99.0% tert‐butylamine selectivity. The effect of reaction conditions on the performance of the
ZSM‐11 catalyst agreed with the thermodynamic results, which provides guidance for further catalyst
development and reaction condition optimization.
Attachment: Thermodynamic study of direct amination of isobutylene to tert-butylamine.pdf (568kB)
This file has been downloaded 266 times
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
| Pages:
1
..
95
96
97
98
99
..
104 |