aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
Research Notes on [Ti(cat)3]*2H Complex
Recently I joined a research program as an undergraduate, under a professor named Christian Brückner (https://bruckner.research.uconn.edu/), regarding an ellusive and important complex called Titanium (IV) Tris-Catecholate Diprotonated.
In the following thread I wish to detail my experiencd from day one to the final day of my research, whenever that may be. I want to show to any other
young chemistry majors or even younger the process of research from my point of view and what one may expect to see. Instead of focusing entirely on
the chemistry, I wish to show the progress of interpreting data and using it to further experiment until we have discovered something useful (or not)
and what I did exactly. So far I have spent 3 months (roughly 12-16 hours a week) working on the project and have finally gained my bearings in the
lab, and my hope is to at least be an interesting read for both the experienced and unexperienced.
In the following thread, I will try to give as much detail as possible including my failures, my miscalculations, my successes and even my professors
commentary. This also includes raw data and my preparation thereof. If anyone has suggestions on the best way to represent or show data, I would be
very grateful, and this also goes for suggestions on what I may be missing or things I should read up on. Any and all help is welcome.
In addition, my professor nominated me for a research fellowship over the summer to continue research without school work hindering my progress. He is
confident that we will publish a paper by the end of this year, so that would be an interesting addition to the discussion.
Right now this is going to be a placeholder until I can properly aggregate my data from the school computers to the cloud, in which I will be showing
everyone what I have done. Expect to see NMR, UV-Vis, IR, Mass Spec, as well as pictures of reactions.
And to close off, here are some neat pictures of the lab and my little work area!
Attachment: My lab bench and basic equipment (3.2MB)
This file has been downloaded 223 times

This is a shared fume hood with a grad student (she helps make sure I dont do something stupid)

Shared work station with quick access to cleaning solvents and sink. There are lots of random but actively used chems right here including Bobbitts
Salt (the famous Dr. Bobbitt from down the hall in the lab, was such a cool dude, RIP)

Oh yeah, and there is a wonderful array of colorful Porphyrins in a back cabinet. Probably over 500 unique porphyrins that were either made by my
professor directly during his time as a grad and post grad or by the hands of graduate students underneath him. I mean colors of the entire rainbow
with so many crazy applications.

Seriously, these are so amazing and blow my mind. If only they werent light sensitive, Id photograph them all.
I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|
Jome
Hazard to Others
  
Posts: 154
Registered: 10-6-2004
Location: Soutwest sweden
Member Is Offline
Mood: desiccated
|
|
I'll be following this, I am about to (re) start university studies myself so an insight into what researchers actually day-to-day do rather than just
the results/data would be valuable!
|
|
|
Boffis
International Hazard
    
Posts: 1887
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi aab18011; catechol or its 3-5-disulphonic acid have long been used as reagents for titanium, giving an yellow or orange colour which I have always
assumed was a titanium complex. Is this what you are trying to isolate in the flesh? I will be watching.
Are those protons labile? That is do they act like and acid and form tricatecholotitanate IV salts?
|
|
|
aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
The Beginnings
Upon the first proper discussion with my professor, we tried to discuss what the importance of the research really was. My professor made it clear
that he done some original research with the current pioneers of metal catecholates (pyrocatechol and related are very important and strong
chelators), and that he hadn't done any in the past 20 years. His idea was to pickup where he and his team left off and try to not only characterize
the [Ti(cat)3)]2H, but to also try and see if we could make a heteroleptic (more than one distinct ligand such as Allyl palladium) titanium
catecholate. This idea likely came from the fact that he has been wanting to find a new method to tie porphyrins to the catechol and use titanium as a
sort of anchor.
From here he asked me to first get familiar with various equipment, but to also do so only when I had samples ready. This way, I wouldnt rush to learn
every instrument and possible waste valuable time, and instead I would only learn machines like NMR once I had a few samples to actual NMR. My first
task was to get to know UV-Vis spectroscopy, as it was the only reliable and easy diagnostic for metal complexes.
My first few weeks I spent messing around with serial dilutions in order to really figure out what was happening, and to try to see if the dissolution
of this compound led to ligand exchange. At this point, using our predetermined system of naming compounds, we dubbed this AB-1-6.
While running various solvents, I got to reading about charge transfer complexes such as LMCT and MLCT. This is sort of my discovery of what I should
be expecting with the spectra:
-Titanium (4+) is a d0 complex ion, which exhibits no allowable d->d transitions. This rules out a CT from this source.
-This species does have 6 highly electronegative sources of electrons (the 6 catechol ligands, with 2 being protonated). This can easily provide a
LMCT band.
-There are conjugated pi systems from the catechol units, with oxygens, thus giving way to pi->pi* transitions. I was not sure exactly how many we
should see, but I assumed at least one.
At this point I was ready to try and look into what my professor had already done in his research. I also looked into another older paper he cited
from 1986, by Borgias et al.
Bruckner et al. - Bis-DMSO,Bis-catechol-titanium (new)
Borgias et al. - 1986 paper
Titanium complex design and synthesis - Bruckner et al. 1998
Before I got to discuss some of my findings, my professor also told me to start making my own sample of the catechol complex as his was quite old. I
followed the Borgias 1986 paper at half scale:

Reaction setup - 2 necked rbf with a pressure equalizing funnel and high power condenser. In the picture there is only Titanium tetra chloride
anhydrous in toluene in the addition funnel, and hot toluene in the rbf. The catechol has yet to be charged as it takes a while to dissolve in cold
toluene.

This is post addition of catechol. Funny, though, as my professor came back from a meeting and had a chuckle when he realized the grad student had
grabbed Tin(IV) chloride instead of titanium. Luckily, the reaction still proceeded, as it chucked out immense amounts of hydrogen chloride gas.

Reaction seemed to go fine, but my professor knew it was definitely different since it wasnt a brick red color as he expected.

Bottle in question haha
The following week I came in and re-ran with fresh titanium tetrachloride:


The apparent red color was very different to that of tin, and thus we knew it was correct. Matched the literature exactly. The sold was dried after
washing with hot toluene and cold/dry ethyl acetate (or ether).

I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|
aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
UV-Vis Spectral Data
Now that I had a fresh sample, I went a head and ran as many uv-vis spectras in different solvents as I could, and used multiple concentrations to
measure the extinction coefficient. My professor had to stop me multiple times as I was being sloppy and using data that resided in the 3-10
Absorbance units region. This data was faulty as the absorbance units are exponential and carry lots of noise. You may be able to see that in some of
the raw data posted below. The super high concentrations (roughly 1*10^-4M) was very noisy. Mind you I heated each solution to dissolve as much as
possible with pipette filtering to get rid of solids.
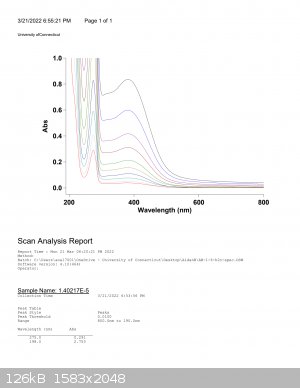
This was the UV-Spectra for water at various concentrations, which came with batch data that I could plug into excel or spreadsheets and do some work
with.
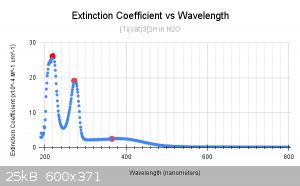
The reason this became our baseline is due to the fact that it is almost totally insoluble in water and does not seem to exchange with water. It may
be exchanging but it doesnt seem that way. So for the rest of the graphs, you will be seeing the fact that the solvents are all compared to H2O.
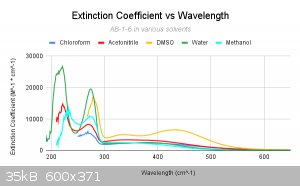
This graph here is nice as it compares all of the main solvents to one another. Now looking at these graphs, we came to the conclusion that many of
the solvents are shifting much, although they seem to be able to somewhat dissolve our complex, whether it be a little or a lot. However, in the case
of DMSO, it seems as though it indeed exchanges as the LMCT band (which now shows as two separate bands) has split and shfited in two sections. We see
the initial bands in the 200-300 range are shifted right (bathochromic shift). Although methanol does not align perfectly, this is likely due to the
fact that it has a weak uv-vis cutoff. This could easily cause the lower end of the spectrum to come out wonky.
From here on out, all of my comparrisons will use the water and these others as baselines.
I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|
aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
Quote: Originally posted by Boffis  | Hi aab18011; catechol or its 3-5-disulphonic acid have long been used as reagents for titanium, giving an yellow or orange colour which I have always
assumed was a titanium complex. Is this what you are trying to isolate in the flesh? I will be watching.
Are those protons labile? That is do they act like and acid and form tricatecholotitanate IV salts? |
I have much data to show you, and hopefully you will have your answer quickly. I have discovered a lot, and more will come. My hint to you in the
meantime is that the solubility will tell us a lot. And catechol has been the traditional tool, but the disulphonic acid has also been used. Many
complexes with these can be made. Im sorry I dont have a quick paper on hand, I have probably forgotten more papers than I remember at this point
haha.
I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|
aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
Moving Forward
Up until this point, I have discussed very little in terms of theory, and I quickly hit a wall. What in the world was I to do next? I honestly began
to realize how quickly I did this work, and I actually forgot to really ponder what I needed to do.
After a few days, I decided to just ask my professor what direction I needed to go in. Being the cryptic man he is, he left me with, "WHERE ARE THE
PROTONS, BRADLEY?" And smiled as he ran out the door with no further explanation. It took me some serious thought, and yet I came to no conclusion.
After debating whether I should work up the courage to go see him and ask again, I finally sucked it up and snuck into his office and waited. It took
about half an hour before he walked in and asked," Well, what have you found..." to which I replied," Nothing, because I feel helpless." After that,
he sat and looked at me until he told me a few new hints. "Have you looked at the crystallographic structure at all, and what about the [carbon] NMR?"
This was all he needed to say, and so he sent me off.
I quickly came to realize that I was missing something within the NMR, as I realized he directly took NMR for his compounds and referred to them in
his own work. So he knew something I didn't. I'll admit I ended up recruiting the help of one of my grad student office neighbor, Dinu, who gave me
some very insightful opinions. She led me to the confusion of NMR signals listed in this literature: Chi et al. 2001. Here they cited that they used a d6-Acetone for an NMR of the tris-catechol. This gave them exactly 3 signals.
When drawing out the possible forms of catechol (unprotonated, diprotonated and monoprotonated forms), I came to the conclusion that we should see
more than 3 if there is some uneven protonation going on, even if one of the three catechols remain totally unprotonated. The only two forms that
could exist with 3 unique signals only would be the totally protonated and the totally unprotonated forms. Since we know that they are indeed
protonated, we must try and figure out why using some geometry. Luckily, my professor gave me a bunch of octahedral papier-mâché sets, so I can mess
around and try and learn something.
 
To me, this is the only way this would work, with the hydrogens being stuck in the trigonal faces (octant) on opposing sides, almost like you could
skewer them through an axis, as seen in their stacked positions above. It also led me to the thought that there is a polymeric-like nature to these,
and since it is so hard to dissolve without some sort of heating that it could even mean that it is a polymer.
One thing I remembered my professor saying was that it had a Bailar-Twist, which allowed it to take on two isomers.

Just click on this, its a svg with zero alpha channel which makes it impossible to see. This rapid switching of isomers in solution could be slowly
quenched by a slow crystallization caused crystals of opposing isomers. But in the case of my red-brick colored product, it is an insoluble power of
fine nature. A lightbulb went off at this time, and I thought that maybe, just maybe, because of the nature of the toluene solvent, there are many
small crystals of isomers which could not form one large crystal due to the intimate face-on-face bonding of the octahedral faces with the proton as
the binder. This would explain why it can dissolve after heating, but not when suspended. But again, the only way to prove this is through some
special polymer analysis, which is too far out of my reach. It will have to be sampled and sent to the polymer lab down the hall.
In the meantime, my job is to try and swap one or more catechol ligands. But my idea is to do it in a way where we force the hydrogen out of the face
of the octahedral. Take a look at where the salt has the hydrogen in this following crystal image. (entry CIGTUH)
In the triethylammonium salt of the compound, not only do the crystal grow pretty long, they grow in nice needles. This is vastly different to that of
the regular diprotonated version. We also see in the crystal image for it that it pulls the hydrogen out of the octant/face of the octahedral, such
that the crystals are more loosely bound. Another difference is the spectral data for this new salt. In the region of roughly 350-400 nm, we see a
much greater absorption there, which leads me to believe that the relief of the proton being pulled out of the interface of the crystals has allowed a
greater charge transfer to exist between the oxygens and the titanium center. Since the hydrogen needs exactly -1 of a charge to satisfy it, we can
say that the positioning of the hydrogen in the interface would mean that each of the three oxygens are donating -1/3 of a charge, and the other -2/3
of a charge is going to the titanium. Once the hydrogen moves away, we can assume that almost all the oxygens charge is now going into the titanium
center, and thus only relying on hydrogen bonds of the ammonium compound and the ionic nature. This new salt is not like the original, which seems
more and more like a polymer by the day. What is funny is that my professor suggested that they are weaker and can be cleaved easily, but seems
convinced that this compound is much stronger than previously thought.
I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|
aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
To finish off this brief summary of where I started to where I am at:
-I need to try and make new bulky salts to try and pull the hydrogen away,
-I want to try and make a diprotonated non-polymerized crystal, that dissolves in water without issue,
-I want to get more solubility data,
-I want to get some characterization of the polymer, and hopefully learn more about its structure and length(s).
With this in mind, there is much work left to do before we can start to just test ligand exchanges so willy nilly. I have some ideas for some ligand
exchanges, after I test the lability of the salt with triethylamine. What if we try the DMSO exchange with the triethylammonium salt? Will it still
exchange, will it exchange faster? Will it fail? If so, what does it mean? So probably tomorrow or even monday, I will be testing this with the
crystals I get.
I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|
DraconicAcid
International Hazard
    
Posts: 4378
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Okay, so the Ti(cat)3H2 complex presumably has one C6H4O2(2-) ligand and two C6H4(OH)O- ligands. The protons on those ligands aren't going to show up
well on X-ray crystallography (unless resolution has improved a lot since I last did a structure), and I'd expect them to get broadened to heck in 1H
NMR.
To me (and bear in mind that I'm not a titanium chemist), the fact that it's far less soluble in water than the triethylammonium salt is simply
because it's a neutral complex rather than an ionic one. It doesn't necessarily mean that it's polymeric.
If you really want to find out where the protons are, I'd suggest making a complex with one equivalent of catechol and two equivalents of
methoxyphenol, and see where the methyls end up, and if that structure is spectrally similar to the one with protons.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
Quote: Originally posted by DraconicAcid  | Okay, so the Ti(cat)3H2 complex presumably has one C6H4O2(2-) ligand and two C6H4(OH)O- ligands. The protons on those ligands aren't going to show up
well on X-ray crystallography (unless resolution has improved a lot since I last did a structure), and I'd expect them to get broadened to heck in 1H
NMR.
To me (and bear in mind that I'm not a titanium chemist), the fact that it's far less soluble in water than the triethylammonium salt is simply
because it's a neutral complex rather than an ionic one. It doesn't necessarily mean that it's polymeric.
If you really want to find out where the protons are, I'd suggest making a complex with one equivalent of catechol and two equivalents of
methoxyphenol, and see where the methyls end up, and if that structure is spectrally similar to the one with protons. |
Its funny you suggested that, I have been trying to come up with other methods of messing around with the protons. My first idea was Dimethoxybenzene,
but I like the monomethoxy better.
All I have to do is discuss with my professor. 
I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|
DraconicAcid
International Hazard
    
Posts: 4378
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Also, if you want to improve the solubility of the neutral complex in organic solvents, slap some alkyl groups on the catechol (probably para to the
hydroxyls, so they don't crowd each other on the complex).
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
aab18011
Hazard to Self
 
Posts: 74
Registered: 11-7-2019
Location: Connecticut, USA
Member Is Offline
Mood: Moving out and setting up shop in my new chemistry hobbit hole
|
|
new visualizations
This file below is the molview representation of my complex, which just so happens to be quite accurate. Judging by the crystallographic image of the
triethylammonium salt of the trie-catechol, this image is structurally sound.
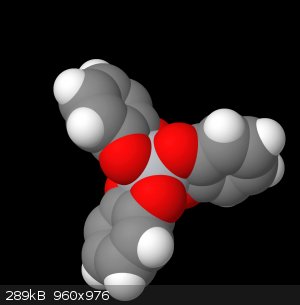
Id like to point to this next image, and I want you to imagine the octahedral face that exists here between these 3 oxygens. The hydrogen sits in this
face, not directly a child of any of the oxygens, rather sharing them equally (which is the reason the NMR isn't picking it up)
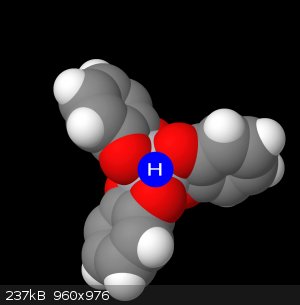
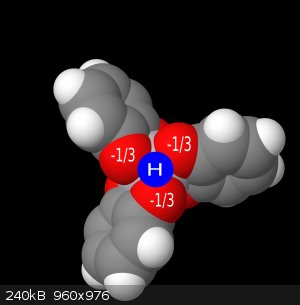
Here I have illustrated below a sort of fractional charge donation, where for each group of 3 oxygens, when the complex is protonated, will donate 1/3
of a charge from each oxygen to the proton, and 2/3 of a charge each to the titanium. This gives us the accurate neutral complex. But this has
important implications in understanding and proving this is actually what's going on because of some UV-Vis spectra I have. The spectra observed for
pure [Ti(cat)3]2H in water vs the [Ti(cat)3]2(HNEt3), shows the LMCT band being more allowed/stronger in the triethylammonium salt. When looking at
the crystallographic image(ccsd-crystallographic, entry CIGTUH), the triethylammonium proton is being held outside of the complex octahedral face and rather directly over a
singular oxygen. This would allow a greater charge donation into the titanium, thus leading to a greater LMCT band.
From here the problem is now about trying to isolate a crystal for X-ray crystallography. This will allow me to better understand whether we have a
polymer or an actual crystal. The issue with traditional characterization is the fact that the some polymers are soluble as well as they don't always
break under dissolution conditions. I am going to talk to some polymer boys down the hall, and hopefully get some alternative methods in case my
methods do not work.
As of today I have started about 6 new crystallizations, 2 which are gas exchange methods, and 4 that are slow cooling crystallizations. I first made
a stock liquor of 20:1 Ethanol to water, and then got it boiling and dissolved around 64 mg of the complex. I then separated it into aliquots in vials
(small vials for the gas exchange as it has to be small enough to fit in a scint vial), as well as filtered using cotton wool in a Pasteur pipette.
Then for the 4 slow crystallizations, I added 1 ml, 2 ml, 3 ml and 4 ml of water to change the ethanol to water mixture. Since each container now has
a different mixture of water to ethanol, we can see what that changes. Its super hard to get a solution with just pure DI water, which leads me to
believe that the water is less basic than the combined oxygen interactions. No singular oxygen is more basic, but together at the octahedral face,
they form a really strong neutral complex.
In addition, I am starting more UV-VIS spectroscopy with 11 new solvents:
-Ethanol
-n-propanol
-iso-propanol
-Octanol
-Ethyl ether
-Ethyl acetate
-THF
-Dioxane (1,4)
-DCM (gave me trouble a while ago)
-Cyclohexane
-Pyridine
Hopefully we can compare solvents that have zero capability of complexation or ligand exchange to solvents that potentially can, which will give us a
nice way to detect complexation changes. This will give us a more accurate baseline.
The crystal image for the triethylammonium complex will be added at the end of this, as it contains a density which can be used to get an idea of how
little compound is needed to get a nice singular crystal (its literally in the 40 micrograms). This allows us to use as little product necessary to
get a crystal. I'd like to point out that this paper says that they couldn't not get any crystals from their attempts with various solvents, including water. But my professor is very wary
and does not entirely believe them. He wishes to at the very least try, and if not, then go back to the drawing board. We discussed exhaustive
methods, mostly the very slow and low method. Possibly Thursday this week (4/28/22) I will be doing the setup for that. I was going to try and use
minimal ethanol to dissolve the complex, and try to dilute it out with water and then set it out to evaporate slowly in a vial in a chamber (to avoid
contamination of the crystals).
We shall see...
Attachment: 1125161.txt (5kB)
This file has been downloaded 225 times
Oh and before I forget, here are the two images regarding the Titanium tri-catechol diprotonated in water and the Triethylammonium titanium
trie-catechol in water.
Attachment: net3-triscat.pdf (184kB)
This file has been downloaded 248 times
Attachment: h2o-triscat.pdf (247kB)
This file has been downloaded 230 times
I am the one who boils to dryness, fear me...
H He Li B C(12,14) Na S Cl Mn Fe Cu Zn Ba Ag Sn I U(238)
"I'd rather die on my feet than live on my knees" -Emiliano Zapata
|
|
|
|