| Pages:
1
..
17
18
19
20
21
..
23 |
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Acting on a suggestion from MineMan, I tested a mix of 85% UZP and 15% Al-powder (nominally 3 um). In my standard setup it gave a dent of 3.71 mm
(picric acid 3.53 mm and RDX 4.13 mm). This is still considerably lower than would be expected from the calculated VOD and Pcj data, but much better
than my previous test with pure UZP which gave 2.92 mm.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1386
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
It are very good results. Powerful than picric acid. Aluminium usually decrease brisancion, but here is it conversely. And the UZP works in your
cavity as primary - secondary ?.... ...... Was tested? I know, you use for
start aminoguanidine some perchlorate + PETN 300 mg for maximal kick of examined compoud. 300 mg PETN is too much. This can affect the result. It's a
huge blow to the test substance. It would make a small dent in aluminum, even if the place of the substance under investigation was pressed sand. Just
my opinion, nothing more. ...... Was tested? I know, you use for
start aminoguanidine some perchlorate + PETN 300 mg for maximal kick of examined compoud. 300 mg PETN is too much. This can affect the result. It's a
huge blow to the test substance. It would make a small dent in aluminum, even if the place of the substance under investigation was pressed sand. Just
my opinion, nothing more.
[Edited on 10-2-2022 by Laboratory of Liptakov]
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I think there is a very high likelihood of it being able to DDT reliably under strong confinement. When heated in folded Al-foil, it tends to "pop"
like flash powder. It is not extremely flame sensitive, but I think it is likely that transition metal complexes might be.
Regarding the use of 300 mg PETN, you are correct that it is enough to slightly dent the Al block even if the base charge is completely inert.
However, since I have plans to also test some things that are very insensitive, and since the experimental setup should be identical in order to
compare the results, I settled on this amount of booster. The point in being careful to use the exact same amount of PETN every time, is to make sure
that it adds the same amount to the dent in all experiments.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1386
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Yes, that makes sense. Promising materials that could be capable of DDT as a stand-alone monopropelant can be tested later.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
MineMan
International Hazard
    
Posts: 1004
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Microtek and LL. Yes it should DDT in strong cavity. That’s has been a thought I have had, although where we want it to shine is the detonation
pressure. From this set up I would have expected a dent of over 4.5mm. Since the Al helped significantly I think this proves your original theory
microtek. Despise the sensitivity it behaves as a non ideal explosive. Unless somehow the detonation wave is dead pressing the UZP ahead of it. But,
the wave should move far faster then the speed of sound in UZP obviously.
I suppose the only other thing to try is compressing it to a density of 1.85-1.9 instead of over 2 or try larger quantity such as 5-10 grams. What do
you think (LL and microtek)
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
The last couple of tests I have done with UZP (with and without Al) have been just hand pressed precisely to avoid the likelyhood of dead pressing, so
I'm afraid there's not much to gain there. However, another thing is that since UZP is so dense, 1 gram of it in a 7mm coloumn is just 13mm long (at 2
g/cc). It may be that the detonation doesn't have time to accelerate to its max velocity in such a short run. After all, when making shaped charges we
make sure to have a certain head height above the apex of the cone. So, I will have to do an experiment with a longer charge. Then I will also have to
do identical experiments with some of the other energetics, to establish a new baseline. To do these, I will have to construct a larger test chamber
(since a longer charge with same diameter will obviously involve more material).
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1386
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Of course, that bigger amount will give more exact results and exact measurement of density. I estimate that 5g will enough. Diameter of high pressed
EM of cylinder should by in this cause 12 - 15 mm at 5 g.
However alu brick must be a lot bigger. Which can be expensive. For 5g I reccomend using lead block of diameter 70 mm and 60 mm high.
Which is possible use repeatedly after casting. And measurement can be on 0,1 mm. Not on 0,01 mm.
Lead block is possible use also for measurement longer charge in cavity 7 mm / 5g. With less exact results. Advantage of lead is his huge dynamic
resistance of entire block.
But 5g require testing chamber minimal 70 x 70 x 70 cm with wet sawdust.
[Edited on 12-2-2022 by Laboratory of Liptakov]
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
MineMan
International Hazard
    
Posts: 1004
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Laboratory of Liptakov  | Of course, that bigger amount will give more exact results and exact measurement of density. I estimate that 5g will enough. Diameter of high pressed
EM of cylinder should by in this cause 12 - 15 mm at 5 g.
However alu brick must be a lot bigger. Which can be expensive. For 5g I reccomend using lead block of diameter 70 mm and 60 mm high.
Which is possible use repeatedly after casting. And measurement can be on 0,1 mm. Not on 0,01 mm.
Lead block is possible use also for measurement longer charge in cavity 7 mm / 5g. With less exact results. Advantage of lead is his huge dynamic
resistance of entire block.
But 5g require testing chamber minimal 70 x 70 x 70 cm with wet sawdust.
[Edited on 12-2-2022 by Laboratory of Liptakov] |
What do you mean dynamic resistance of lead is huge?
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1386
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Lead is heavy. Test not need any solid pad for same repeatedly results. Lead block can be in any position at the test in chamber or sand pit. And
"swim" there. Other on picture. For funny is there production special spoon for slow eating of soup.....

Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
MineMan
International Hazard
    
Posts: 1004
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Laboratory of Liptakov  | Lead is heavy. Test not need any solid pad for same repeatedly results. Lead block can be in any position at the test in chamber or sand pit. And
"swim" there. Other on picture. For funny is there production special spoon for slow eating of soup.....
|
That’s the only way I eat my soup. But I got a patent pal and my lawyer is gonna give you a call
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1386
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Feel free to patent the hole spoon . By your advice (for UZP) was tested Lithex with 10% aluminium powder bright. Interesting is, the deep is exact
same as without aluminium. 7,5 mm exactly. Complete energy of detonation was bigger according flying sawdust and other flying parts of testing
chamber. Aluminium can provide advantage used less amount of Lithex for a same brizancion. And also for somes application, where is total energy
importantly, than maximal brizantion. For example EFP not require maximal brizantion, there is more importatly maximal energy for better shaping of
slug. Was observed also more easy filling - pressing behavior, resoectively pull of rod. If is Aluminium not critical compound, he provide clear
advantage in mixture Lithex. Interestling...
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
MineMan
International Hazard
    
Posts: 1004
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Laboratory of Liptakov  | Feel free to patent the hole spoon . By your advice (for UZP) was tested Lithex with 10% aluminium powder bright. Interesting is, the deep is exact
same as without aluminium. 7,5 mm exactly. Complete energy of detonation was bigger according flying sawdust and other flying parts of testing
chamber. Aluminium can provide advantage used less amount of Lithex for a same brizancion. And also for somes application, where is total energy
importantly, than maximal brizantion. For example EFP not require maximal brizantion, there is more importatly maximal energy for better shaping of
slug. Was observed also more easy filling - pressing behavior, resoectively pull of rod. If is Aluminium not critical compound, he provide clear
advantage in mixture Lithex. Interestling... |
Interesting test indeed! I am not surprised. Al always helps DDT . Now if you can source nano 100nm Al you will find your Lithex will DDT in
50-100mg, perfect for micro detonators.
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
In case someone wants to experiment with urazine for themselves, I'm attaching the relevant pages from Inorganic Synthesis vol 4 here:
Attachment: Urazine pages.pdf (332kB)
This file has been downloaded 1149 times
|
|
|
MineMan
International Hazard
    
Posts: 1004
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Thank you Microtek! Any updates on the application of Urazine?
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I did a quick test of the use of urazine as a ligand in a Ni2+ complex with perchlorate anions. I dissolved Ni(NH3)6(ClO4)2 in hot water, added a
little extra HClO4, and then gradually added two equivalents of urazine. This produces a very insoluble precipitate, that is only very slightly
energetic. Wrapping it in Al foil and heating in a flame gives only a minute expansion of the foil package.
My hypothesis is that the precipitate is maybe nickel urazinate and not a complex at all.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1386
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Lithex is in a similar situation. Enclosed in foil during heating, in the end it always just burns. And still quite reluctantly.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Recently, I have devoted my spare time to developing test methodology for measuring VOD using an oscilloscope. I can now report my preliminary
findings:
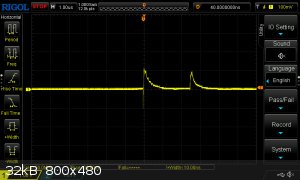
This is a screencapture from a test on pure PETN with probe placement 15 mm apart. The probes are ionisation probes, with each probe simply consisting
of two square cut copper leads inserted (the picture shows a single probe point):

The test sleeve is resin 3d printed and the flaring base is just to have a secure attachment to the print plate. It also allows me to glue it onto a
witness plate if I want to test brisance along with VOD (the axial hole goes all the way through). The picture doesn't do it justice, but the probe
holes are very cleanly printed at exactly 5mm center distance.
The principle of this testing method is that the reaction zone just behind the detonation front is conductive since it is a high density plasma.
Therefore, it can act as a switch to close a circuit. I have then constructed a simple circuit with the probes acting as switches in parallel and
included a voltage divider setup to allow the change in signal to be detected, even if the conduction zone hasn't completely faded at one probe before
triggering the next. The circuit is powered by a 9V battery, which provides the signal.
In the screen capture above, you can clearly see the very sharp rise in the signal as the detonation reaches the probe, followed by a gradual fall as
the conductive zone attenuates. The time between the leading edges is very close to 2.5 us which, with probes 15mm apart, corresponds to a VOD of 6.0
km/s. It is difficult to assess this value since I don't know the exact density of the charge. Also, litterature values are often the result of tests
on much larger charges, so even if I did know the density, the VOD of my charge would be less than most litterature values at the same value.
Nevertheless, I find the results encouraging.
I still have much work to do, examining the reproducibility of the setup and trying more than two probes to see if I can map the velocity profile. I
also have to examine many different explosives to see if the detonation zone is similarly conductive for others.
[Edited on 8-3-2022 by Microtek]
|
|
|
Nitrosio
Hazard to Self
 
Posts: 57
Registered: 31-3-2018
Member Is Offline
Mood: No Mood
|
|
Attachment: BCHMX.pdf (1MB)
This file has been downloaded 382 times
Attachment: BCHMX2.pdf (750kB)
This file has been downloaded 342 times
Attachment: BCHMX3.pdf (1.2MB)
This file has been downloaded 346 times
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I think BCHMX is mainly interesting for thos who don't have access to acetic anhydride. If you can get just a small amount of Ac2O and P2O5, you can
go the DADN -> SOLEX -> HMX route. All of the steps are easy, and gives essentially 100% yield. Look for the patents by Lukasavage.
|
|
|
Nitrosio
Hazard to Self
 
Posts: 57
Registered: 31-3-2018
Member Is Offline
Mood: No Mood
|
|
Glyoxal + (Amino)-Guanidine + Tetrazole... ???
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Take a look at this paper. The perchlorate salt is especially interesting IMO: d=1.99 (measured), VOD=9500 m/s and Pcj=45.7 GPa (Explo 5), Tdec=275C,
IS=24J, FS=90N.
The friction sensitivity is perhaps a little high, but other than that, it is almost perfect. The question is if it is hygroscopic like so many other
promising perchlorate salts.
Attachment: azotriazole salts.pdf (539kB)
This file has been downloaded 327 times
|
|
|
MineMan
International Hazard
    
Posts: 1004
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Microtek  | Take a look at this paper. The perchlorate salt is especially interesting IMO: d=1.99 (measured), VOD=9500 m/s and Pcj=45.7 GPa (Explo 5), Tdec=275C,
IS=24J, FS=90N.
The friction sensitivity is perhaps a little high, but other than that, it is almost perfect. The question is if it is hygroscopic like so many other
promising perchlorate salts. |
What is the synth like? Seems expensive involving silver?
Any update on the Urazine?
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
The silver is not really necessary, just convenient, and would be recycleable anyway. The synthesis involves oxidative coupling using sodium
dichloroisocyanurate (ordinary pool chem) and then reaction with triphenylphosphine which I need to buy.
Lately I have been working on The VOD measurements, so I haven't experimented further with urazine.
|
|
|
MineMan
International Hazard
    
Posts: 1004
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Thank you Microtek!
|
|
|
greenlight
National Hazard
   
Posts: 737
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Full-nitro-nitroamino cooperative action: Climbing the energy peak of benzenes with enhanced chemical stability:
https://www.science.org/doi/10.1126/sciadv.abn3176
Here's the paper with the actual synthesis notes for TNTNB if interested:
Attachment: sciadv.abn3176.pdf (1.6MB)
This file has been downloaded 305 times
[Edited on 29-3-2022 by greenlight]
Be good, otherwise be good at it 
|
|
|
| Pages:
1
..
17
18
19
20
21
..
23 |