karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
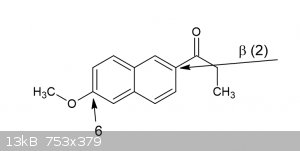
regioselective red. electrophilic 6-demethoxylation of ß-naphthalene derivate
Ok so I have this compound, its a ketone actually(a beta-propionaphthone), and in case I would want to remove the 6-methoxy group, and given its very
similar characteristics thanks to its position to the 4-methoxyphenyl group, what speaks against the attached procedure working analogous for this
analogue?
It seems logical to me, but I probably miss something.
The paper is one of the Azzena et al papers, where they react 3,4,5-trimethoxybenzaldehyde (and trimethoxyphenylalkyl ketones, in follow up papers),
as their acetals with metallic sodium in THF, then react that with an electrophile, which can also be water, and thus resulting in cleavage of the
4-methoxy group.
I know I could remove that group else, but it would be more work and thus easier to simply acylate naphthalene without the methoxyether from the start
on.
If this could work that simple, it would at least warrant the one or other experiment.
Attachment: jo00037a029.pdf (837kB)
This file has been downloaded 205 times
verrückt und wissenschaftlich
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 200
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
What exactly is the compound you have? you say you have a beta-propionaphthon (presumably the first beta, pos 2), but then a methoxy you want to
remove on 6. Isnt that then quite unlike the 4-methoxy benzaldehyde? or are you thinking the resonance will allow it to behave the same? worth a shot
IMO.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2799
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
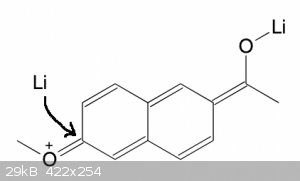
In principle, yes, although the resonance will be weaker with naphthalene because the whole naphthalene is involved. So not sure if it will work.
But I thought the binding data said the 6-methoxy was fine?
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Quote: Originally posted by clearly_not_atara  | | In principle, yes, although the resonance will be weaker with naphthalene because the whole naphthalene is involved. So not sure if it will work.
|
Oh, that is interesting via enolate!
Guess I will try that too 
Binding data for exactly those will be had in a while, not yet though 
The chinese had a few and hard to dig up, but of the weaker analogues.
First bioassays were not strongly convincing, but nothing was tried in depth yet, and then again, only a little bit of the pyrrolidine and the
dimethylamine, and there is so much more to do.
I just have a whole lot of this stuff and I am considering all options to try out with it 
I've even coupled this already via bromoketone with 1-benzylpiperazine, (apparently a precursor for a class of triple reuptake inhibitor
antidepressants... not with the carbonyl, who knows  ) )
verrückt und wissenschaftlich
|
|
|