superamoeba
Harmless

Posts: 3
Registered: 30-8-2019
Member Is Offline
|
|
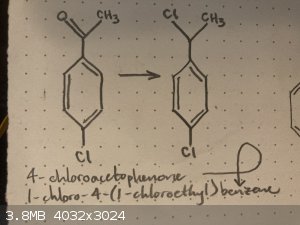
Halogenation of 4-chloroacetophenone
I am trying to make 1-chloro-4-(1-chloroethyl)benzene, and I thought that 4-chloroacetophenone would be a good starting material. However, I am only
a first year chemistry student and I am still learning a lot, so I’m a little bit unsure of the best way to go about conducting the halogenation.
If anyone here has any ideas of a good way to do this, please let me know, I would greatly appreciate any input.
Thank you
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Halogenation?
Read up on alpha-halogenation of carbonyls first.
I prefer to use NBS for that, with a dash(10% molar of the NBS) of tosic acid as catalyst.
That gives in a melt quantitative yields for C2 aromatic ketones like acetophenones are.
Then, you need to remove the carbonyl (wolff-kishner maybe, or in two to three steps via reduction to the carbinol first and then to the alkane, with
whatever is suitable, e.g. acetylation of the carbinol to the followed by reduction).
Honestly, I'd rather use another starting material instead.
Or just buy the para-chlorophenylethyl chloride... its a cheap chemical after all.
For example, para-chlorostyrene would also be suitable as starting material.
The halohydrin would be first formed, and then the carbinol reduced as above.
verrückt und wissenschaftlich
|
|
|
Dr.Bob
International Hazard
    
Posts: 2748
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
I would just reduce the carbonyl to the alcohol and then stir with HCl. Might take a while or a couple of passes to exchange the benzylic -OH for a
Cl-, but I think it is doable, if not use a harsher chlorinating agent like thionyl chloride.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Agreed. Reduce with borohydride to the alcohol, and then cold concentrated hydrochloric acid will give you the benzyl chloride.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Uhm guys, but its an acetophenone?
Reducing the carbonyl gives 1-(p-chlorphenyl)ethanol and not the benzyl alcohol.
And secondary alcohols chlorinated with just HCl?
Maybe if you do it lucas-style with some ZnCl2... at they hive, they did that with pseudoephedrine even.
Thats not was OP was after, so anyway.
But I agree with you that it would be that simple in case of the 2-(p-chlorophenyl)ethanol.
verrückt und wissenschaftlich
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by karlos³  | Uhm guys, but its an acetophenone?
Reducing the carbonyl gives 1-(p-chlorphenyl)ethanol and not the benzyl alcohol.
And secondary alcohols chlorinated with just HCl?
Maybe if you do it lucas-style with some ZnCl2... at they hive, they did that with pseudoephedrine even.
Thats not was OP was after, so anyway.
But I agree with you that it would be that simple in case of the 2-(p-chlorophenyl)ethanol. |
I don’t
understand what you’re talking about. The acetophenone should be reduced to the secondary, benzylic alcohol… since it’s a benzylic alcohol, the
corresponding carbocation should be quite stable, so chlorinating it with HCl is totally plausible.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2748
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
I have actually done exactly that type of reaction before, and it works even better with the extra methyl, as more like a SN1 reaction, in fact you
get traces of the alkene in the product, which can be a pain. I usually stir it neat (vigorously) overnight with an equal amount of Conc HCl, then
separate the phases and react it with a fresh batch of HCl, as once you get more than a small amount of excess water, the reaction stops, so doing it
in two runs gets a higher yield (about 90% on first wash, over 95% on the second run). Once it is done, you can add some hexanes to better separate
the phases, and then you can dry the hexanes layer with Na2SO4 or CaCl to remove any remaining water. I have done it on a 100g scale. You can also
just dissolve it in DCM and bubble through HCl, that may give even better yields, but is more painful to setup. I hate having to use gas cylinders
if I can avoid it.
|
|
|
superamoeba
Harmless

Posts: 3
Registered: 30-8-2019
Member Is Offline
|
|
Thank you, everybody for all of your helpful suggestions, I think they should definitely get me started and give me something to work on in the near
future 
Just as a related question, could I also synthesize 1-bromo-4-(1-chloroethyl)benzene from 4-bromoacetophenone in a similar fashion by reducing with
NaBH4 and chlorinating with HCl?
[Edited on 17-2-2022 by superamoeba]
[Edited on 17-2-2022 by superamoeba]
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Yes.
Reflux condenser?? I barely know her!
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Damn, no more posting without glasses for me, my bad and apologies for that 
verrückt und wissenschaftlich
|
|
|