CycloRook
Hazard to Self
 
Posts: 89
Registered: 2-4-2018
Member Is Offline
Mood: No Mood
|
|
Choosing reflux condensers ?
Does anyone have a system for choosing reflux condensers ?
I'm assuming their is a rule for type of solvent volume being refluxed etc.
How to ensure you don't overwhelm the condenser.
Thanks
|
|
|
crow6283
Hazard to Self
 
Posts: 60
Registered: 14-1-2018
Member Is Offline
Mood: No Mood
|
|
Well the general rule is simply the more volatile, lower the boiling point, the more efficient a condenser is needed. Lower boiling point solvents
like DCM, diethyl ether, even methanol I use a dimroth/coil type condenser. Most anything else a Liebig. For the really low boiling ones I often use
a cooler with plenty of ice in it and a pump for circulation rather than going from the tap - unless it’s winter.
Maybe there is some rule or chart out there but to me it came from experience (failure or two) and then adjusting as you go.
Better safe than sorry though, right ? When in doubt I go for a more efficient condenser.
|
|
|
Llamamide
Harmless

Posts: 2
Registered: 30-4-2019
Location: Germany
Member Is Offline
|
|
Organicum; Practical Handbook of Organic Chemistry, 1st English ed.; Becker, H., Ed.; Addison-Wesley Pub. Co: Reading, Mass, 1973.
| Quote: |
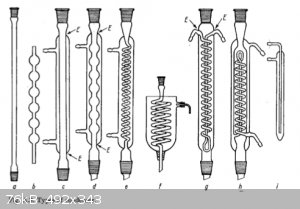
air condenser (a). Because of the low cooling effect of the air, (a) can be used only for high-boiling substances with boiling points
over 150°C. It is sometimes used as a reflux condenser in the form of a "vertical tube", but is not very efficient for this purpose because laminar
flow prevails and the substance easily "breaks through". The modification (b) is more suitable as a reflux condenser, and is used particularly in
semimicro preparations where, because of the small amounts of heat to be removed, air cooling is frequently sufficient even with low-boiling materials
(where necessary, the condenser may also be wrapped with moistened filter paper or a damp cloth). With rates of distillation that are not too high,
(a) can also be used from about 150°C as a product cooler for high-boiling substances.
Liebig condenser (c) is used mainly as a product condenser (up to about 160°C). The cooling medium is flowing water up to about
120°C and stationary water from 120-160°C. Because of the small cooling surface and the laminar flow, the Liebig condenser is not very effective as
a reflux condenser and can be used only for relatively high-boiling substances (b.p. > 100°C).
bulb condenser (d) is used only as a reflux condenser. Because of the bulbs the vapour flow becomes turbulent and the cooling effect
is considerably better than that of the Liebig condenser. Since atmospheric humidity deposits on the outer wall (see above), the positions of sealing
are again points of danger.
Narrow coil condensers (e) should never be used as reflux condensers, because the condensate cannot flow away satisfactorily in the
narrow coil and is often thrown out of the top of the condenser, which may give rise to accidents. In the vertically descending position, however, the
coil condenser is an outstanding product condenser and is used particularly for low-boiling substances.
Städeler condenser (f), the cooling vessel of which can be charged with mixtures of ice and common salt, carbon dioxide and acetone,
etc., so that even very low-boiling substances can be condensed in this way.
Dimroth condenser (g) is a reflux condenser with an intensive action. If the relatively large amounts of distillate which adhere to
the cooling coil can be discarded, it can also be used as a product condenser. The points of sealing (E) are outside the zone with a high temperature
gradient, so that the condenser can be used without special precautions up to 160°C.
jacketed coil condenser (h) is a combination of the Liebig condenser and the Dimroth condenser. Since the cooling effect is very
good, low-boiling solvents (e.g. ether) cannot easily escape.
Suspended condensers, cold fingers (i): this special form of reflux condenser can be hung loose in a reflux apparatus and is
particularly useful in semimicro apparatus.
|
Some interesting-to-know parts omitted for better readability. The book is available for free downloads at various places in the internet  I haven't read Vogel's, but I'd assume it would have a similar part about laboratory
glassware and how to use it? Textbook knowledge is not all bad! I haven't read Vogel's, but I'd assume it would have a similar part about laboratory
glassware and how to use it? Textbook knowledge is not all bad!
In terms of not overloading your condenser: do not apply excessive heat and keep runaway reactions under control by cooling or not letting them happen
in the first place. Also be resourceful and learn to do chemistry on the smallest scale possible. Prevents and contains a lot of nastiness.
[Edited on 10-1-2022 by Llamamide]
|
|
|
teodor
National Hazard
   
Posts: 876
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
I used a Dumroth condenser (Duran, with tap water) for temperatures up to 213C. To check the effectiveness I count how many turns of the spiral are
fogged up. Based on this I can say it is not effective for diethyl ether but is quite effective for methanol and everything with a higher boiling
point. For reflux (unlike high-speed distillation), I don't need a big amount of vapors passing into the condenser so I can always regulate my
heating. When I see the visible puffs of vapor coming inside the condenser that means it is already being pushed a bit.
|
|
|
dawt
Hazard to Self
 
Posts: 74
Registered: 9-5-2016
Location: EU
Member Is Offline
Mood: fluorescent
|
|
I really just use three condensers - a 30 cm Dimroth for reflux, a 40 cm Liebig (actually more of a West condenser) for Distillations and a 20 cm
intensive condenser for anything involving DCM or ether (plus a bunch of ice in my water). For small scale anything I also have a 20 cm Liebig in
NS14. This has basically worked for me since forever, never had issues with that system.
|
|
|
CycloRook
Hazard to Self
 
Posts: 89
Registered: 2-4-2018
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by dawt  | | I really just use three condensers - a 30 cm Dimroth for reflux, a 40 cm Liebig (actually more of a West condenser) for Distillations and a 20 cm
intensive condenser for anything involving DCM or ether (plus a bunch of ice in my water). For small scale anything I also have a 20 cm Liebig in
NS14. This has basically worked for me since forever, never had issues with that system. |
what is an
intensive condenser ?
Do you have a picture? I think DCM and Ether are probably the most volatile substances one would encounter for a reflux.
|
|
|
Llamamide
Harmless

Posts: 2
Registered: 30-4-2019
Location: Germany
Member Is Offline
|
|
h) is an "Intensivkühler"
|
|
|
Mateo_swe
National Hazard
   
Posts: 541
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
So a intensive condenser is a jacketed reflux condenser (ie. an ordinary reflux condenser(g) with an outer jacket cooling also the outer surface.
I have a double jackeded condenser (liebig condenser with extra outer jacket), this variant is also quite common and quite effective.
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
I don't think the term intensive condenser is used much in English though.
Don't recall what is used though. I'd just list them as "coil and tube condenser".
The Stadeler condenser is good for low boiling solvents but a pain to clean. (and needs recharging)
For Low boiling stuff I usually use a Dewar condenser with ice/salt if I'll be around to keep an eye on it.
An insulated lid fabricated to fit the top makes the coolant last a bit longer.
There's an awful lot of room for personal preference here
As long as it cools well enough and doesn't choke most things will work.
Ones that have their own little reservoir obviously need more watching than ones you pump water through, but are easier to use with low temperature
cooling mixtures.
(Pumping ice/salt through a Liebig or West condenser leads to an awful lot of condensation in humid climates unless you insulate them)
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 200
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
a while back i did some calculations while distilling a large amount of toluene and acetone.
From my estimations and calculations, my 300mm 24/40 alihn condenser (effectivly ~350mm liebig) can handle 300W of condensation at a temperature
difference of 80C. With some simplifications and completely ignoring pump throughput (which should be far above distillate, so doesnt rly matter),
that gives
Q=10.7 * L * dT
(L=condenser length in meters, dT = temperature difference between vapor and condenser water)
ofc youd have to figure out the Q value yourself, which depends on the vapor flowrate, but if you just want a rough guess then ~50% of the hotplate
power if its around 100C vapor, 80% if its around 40C.
|
|
|