Romix
Hazard to Others (Literally)
  
Posts: 483
Registered: 19-6-2015
Member Is Offline
Mood: No Mood
|
|
monoamide of terephtalic acid
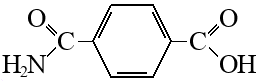
How to make a monoamide of terephthalic from terephthalic acid?
|
|
|
Monoamine
Hazard to Others
  
Posts: 168
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
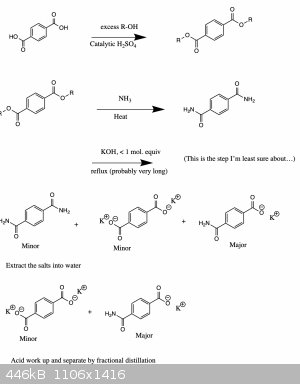
Theoretical attempt
Ok here is how I might try it. No clue whether it would properly work though... Still, it's fun to think about. 
[Edited on 29-11-2021 by Monoamine]
|
|
|
Monoamine
Hazard to Others
  
Posts: 168
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
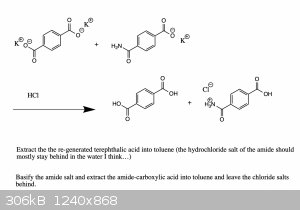
To be fair though, I'm also not sure about the last step (purifying by fractional distillation, since the boiling point of these compounds will likely
be even greater than 300oC).
Maybe this might work as a feasible purification method (?) :
|
|
|
MichaelBijanAfghani
Harmless

Posts: 19
Registered: 15-11-2021
Member Is Offline
|
|
here's another idea:
activating with 1 mol equiv of N,N-Carbonyldiimidazole
Acid base extraction should be able to isolate the mono-activated form from non-activated and di-activated forms.
then the imidazole ester can be reacted with an amine to form an amide.
Details of N,N-Carbonyldiimidazole activation are in are in H.A. Staub et.al, "Azolides in Organic Synthesis and Biochemistry"
I don't have that book right now.
Michael Bijan Afghani
Edit: I'm not sure about the acid base extraction. The mono-imidazole would have one acid and one base group. I wonder if it would be a zwitterion at
pH 7.
[Edited on 1-12-2021 by MichaelBijanAfghani]
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
You might, and this is a stretch, be able to produce a monoester by adding methanol slowly to prevent double esterification. Once esterified, you
could form the amide. Honestly, you would have better luck starting from something like para-methylbenzoic acid. Amidify this first, and then oxidize
the para methyl to the other carboxylic acid.
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Have you thought about preparing the monoamide from the diamide? The latter can be prepared from PET bottles and ammonia fumes. The later material is
probably not very pure but probably adequate for this preparation. I have not tried it but I would treat the crude diamide with 1 molar equiv. of 50%
sodium hydroxide in a stainless steel vesicle and heat until ammonia evolution ceases, leach with warm water (about 20ml per gram), filter off any
unreacted diamide and crud, carefully acidify to pH 2-3 cool and filter of the free terephthalic acid/terephthalic monoamidic acid mixture. If you
plan to carry out a Hoffmann rearrangement to aminobenzoic acid I would carry it out on this material directly and then make it strongly acid to ppt
the terephthalic acid but keep the aminobenzoic acid in solution. Then add sodium acetate to ppt the aminobenzoic acid. Just an idea!
|
|
|
Texium
|
Thread Moved
25-7-2022 at 12:43 |