Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
Neuraminidase Inhibitor Synthesis
With a likely possibility of an influenza epidemic starting from the nearly worldwide H5N1 virus, I have done a large amount of research relating to
the synthesis of neuraminidase inhibitor drugs, which prevent the influenza virus from leaving infected cells. Currently, my effort has been focused
on oseltamivir phosphate, sold commercially as tamiflu, since it is effective against H5N1 and information was fairly available. It now appears that
their are strains resistant to oseltamivir however, so I've started to research other drugs as a contingency plan.
Feel free to discuss other NA inhibitors here, and other related topics. For precursors, try not to dicuss them here if they have another thread
dedicated to them.
Here is what I have so far. All of the chemicals in the procedure are actually doable OTC, but I have not yet typed it all up yet. Search rhodium,
SM, orgsyn and the hive (helps if you have archives of rhodium and the hive) for details until then.
One thing I didn't see there is making acetonitrile by dehydrating acetamide with thionyl chloride. That would be the easiest otc route, since
thionyl chloride is needed in the synth anyway. I think vogel might have a procedure....
Here is the draft of my work so far.
Original procedures that this is based on are found in US patents 6,797,832 and 5,859,284
I put a bunch of refs at the end of the write up. They probably explain all the steps.
For the process based off the first patent, all the solvent volumes are decreased because, compared to the second patent, it seems they went solvent
happy being in a well equipped lab and not trying to do anything on any sort of useful scale.
Advantages of my synthesis:
-All starting chemicals are doable OTC for a determined chemist (and since this could be a life or death thing, the chemist is most likely quite
determined)
-Unlike the patented procedures, no use of reduction hydrogenation and expensive catalysts for making the drug (you'll need to do a raney nickel
hydrogenation for making tetrahydrofuran)
-No obscure chemicals needed for the procedure, anyone here determined enough should be able to pull this off
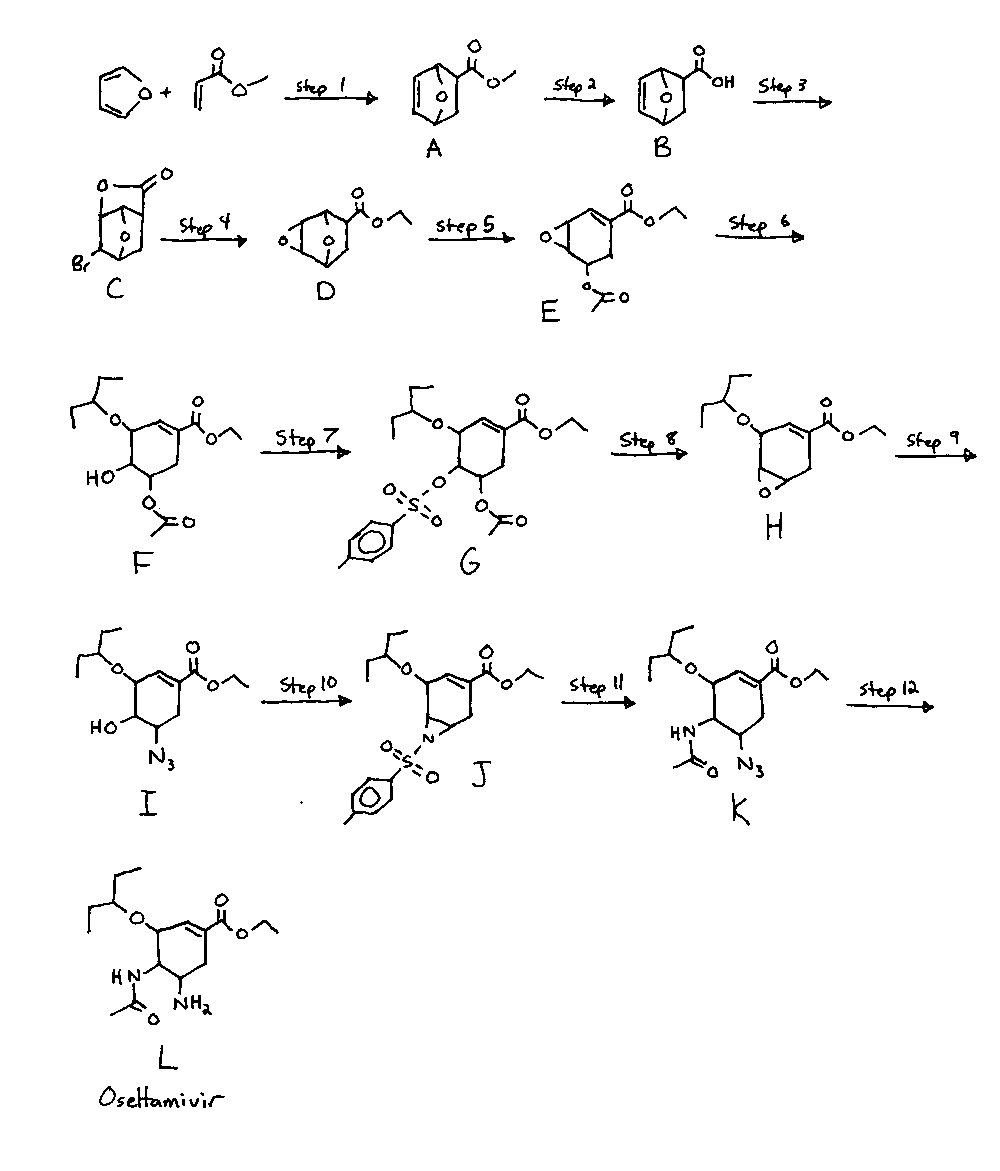
See the attached pic for a synth diagram, so that all my chemical labels ('A', 'D', etc) make sense.
OTC Oseltamiver Synthesis
By Chris The Great
2006
This document has not yet been tested to ensure that it works as presented.
Step One: Synthesis of A
Methyl acrylate (2.78mol) is added to furan (3.95mol) and cooled to -10*C. AlCl3 in DCM (1.15mol in 1-5M conc.) is added slowly with stirring,
keeping the temperature below -10*C. The mixture is then cooled with a cold water bath and stirred for 2 hours. The mixture is added to 1000ml of
saturated sodium bicarbonate solution, and the precipitate is filtered out. The aqeous layer is extracted twice with 250ml DCM, and the organic
layers are combined and dried over anhydrous sodium sulfate. The solvent is distilled away and the product purified by silica gel column
chromatography to give the desired product in 38.6% yield (1.07mol).
[It is likely it can be purified through recrystallization but I have found not details on this. In all likelyhood the unwanted optical would have
similar solubility which complicates things.]
Step Two: Synthesis of B
A (1.07mol) is added to NaOH (1.65mol, 3 to 15M concentration) in water with stirring, maintaining a temperature of 5*C. The mixture is allowed to
warm up over a period of 30 minutes with stirring, and stirred at room temperature for 2 hours. HCl (310ml of 31% sol.) is added to adjust the pH to
below 1, and then the mixture is extracted 4 times with 200ml of DCM. The combined organic layers are dried with Na2SO4, and solvent evaporated and
the product recrystallized from ethyl acetate to give the desired product in 87.5% yield (0.936mol).
Step Three: Synthesis of C
B (0.936mol) is dissolved into 850ml water, and NaHCO3 (0.936mol) is added slowly, with stirring to control the foaming. After foaming ceases,
bromine (0.936mol) is added dropwise with stirring, and then the mixture is stirred at room temperature for 2 hours. The mixture is extracted with
400ml ethyl acetate, washed with 100ml thiosulfate solution (to remove unreacted bromine) and dried with Na2SO4. The solvent is distilled away and
the product purfied by recrystallizing from ethyl acetate, giving the desired product in 89.6% yeild (0.84mol)
Step Four: Synthesis of D
Potassium hydroxide (2.5mol) is dissolved into dimethylacetamide (1000ml) and methanol (150ml), and C (0.84mol) is added slowly as the mixture is
heated to a gentle reflux. It is stirred under reflux for 3 hours, then ethyl iodide (1.6mol) is added and the mixture stirred under reflux for
another 3 hours. To the mixture is added HCl (300ml 31%) and the solution extracted with ethyl acetate (250ml) eight times. The organic layer is
washed with saturated brine and dried with Na2SO4. The solvent is distilled away and the product purified by recrystallization to give the desired
product in 95.9% yield (0.804mol).
Step Five: Synthesis of E
A solution of sodium methoxide (0.9mol) in tetrahydrofuran (500ml) is cooled to -30*C, and D (0.804mol) in tetrahydrofuran (500ml) is added dropwise
with stirring. After the addition, the mixture is stirred at 30*C for 1hr. To the mixture is added acetic anhydride (1.05mol) and the mixture is
stirred at room temperature for 3hr. To the reaction mixture is added saturated ammonium chloride (1000ml), and the mixture is extracted with ethyl
acetate (800ml). The organic layer is dried (Na2SO4) and the solvent distilled away. The product is recrystallized (ethyl acetate) to give the
desired product in 80.0% yeild (0.644mol).
Step Six: Synthesis of F
E (0.644mol) is dissolved into 3-pentanol (600ml) at reflux, and AlCl3 (0.7mol) is slowly added with stirring. The mixture is stirred at reflux for 1
hour, and then allowed to cool to room temperature. A saturated solution of NaHCO3 is added (1000ml), and the mixture is extracted with 750ml DCM,
washed with saturated brine (750ml) and dried with Na2SO4. The solvent is distilled away and the product recrystallized (DCM) to give the desired
product in 81.2% yield (0.522mol)
Step Seven: Synthesis of G
F (0.522mol) is dissolved in DCM (750ml) and triethylamine (0.78mol) is added. The mixture is stirred at room temperature for 5 min. and then cooled
to 0*C. To the mixture is added tosyl chloride (0.78mol) and the mixture is stirred at room temperature for 1hr. The reaction mixture is washed with
saturated sodium bicarbonate solution (1500ml), saturated brine (750ml) and then dried with sodium sulfate. The solvent is distilled off, and the
crude product recrystallized from DCM to give G in 97.3% yeild (0.508mol).
Step Eight: Synthesis H
G (0.508mol) is dissolved in ethanol (1000ml) at reflux, and potassium carbonate (0.25mol) is added with stirring. The mixture is stirred at gentle
reflux for 1.5 hours, and then cooled to room temperature. Saturated ammonium chloride solution (1000ml) is added. The mixture is extracted with DCM
(750ml), washed with saturated brine (1000ml) and dried (Na2SO4). The solvent is distilled away and the crude product recrystallized from DCM to give
H in 98.5% yeild (0.5mol).
Step Nine: Synthesis of I
H (0.5mol), sodium azide (0.6mol) and ammonium chloride (0.6mol) are dissolved into water (70ml) and ethanol (250ml), and refluxed for 8 hours.
Aqeous NaHCO3 (105ml of 8% solution) is added and the ethanol distilled in vacuum. The aqeous residue is extracted with ethyl acetate (250ml), the
extract washed with water (125ml). The wash is back extracted with 125ml of ethyl acetate and the combined organic extracts washed with brine
(125ml), dried over Na2SO4, filtered and concentrated in vacuum to give I as a dark brown oil in 102% yield (0.512mol)
Step Ten: Synthesis of J
Step One:
J (0.512mol) and ammonium chloride (1.2mol) are dissolved into ethanol (1500ml), and zinc (0.7mol) is added and the mixture refluxed for 30 minutes.
The solvent is distilled off to give Ib, which is used directly in the next step.
Step Two:
Tosysl Chloride (1.13mol) is added portionwise at room temperature to a stirred mixture of Ib (from the step one), K2CO3 (2.5mol) in acetonitrile
(1000ml) and stirred for 6hr. Toluene (2500ml) is added, the solid is filtered off and the solvent evaporated to give J in approx 80-90% yeild. It
is used directly in the next step without purification.
Step Eleven: Synthesis of K
J (0.494mol), sodium azide (1.2mol) and ammonium chloride (1.2mol) in dimethylacetamide (400ml) is heated to 80-85*C for 5 hours. NaHCO3 (50mmol) in
water (250ml) is added, and the mixture extracted with hexanes (6x250ml). The combined hexane extracts are concentrated to 1200ml, and 250ml DCM is
added, followed by 1100ml NaHCO3 8% solution and acetic anhydride (0.6mol). The mixture is stirred at room temperature for an hour, and then the
aqeous layer is removed. The organic phases are concentrated in vacuum to 430g total weight), dissolved in ethyl acetate (50ml). The mixture is
cooled and K crystallizes out and is collected by filtering. The crystals are washed with cold 15% ethyl acetate in hexane (250ml) and dried in a
vacuum at room temperature, to give K in 55% yield (0.272mol). [may be slighly higher, ie 60%]
Step Twelve: Synthesis of L (oseltamivir freebase)
K (0.272mol) is dissolved into ethanol (600ml) along with ammonium chloride (0.64mol). Zinc (0.36mol) is added and the mixture refluxed for 30
minutes. The precipitate is filtered out, giving L in 98% yeild (0.266mol). [90-95% more likely]
Step Thirteen: Synthesis of Oseltamivir Phosphate
L (0.266mol) is dissolved in acetone (1000ml) and treated with phosphoric acid (85%, 0.266mol) in absolute ethanol (300ml). The mixture is cooled,
and after 12 hours the precipitate is filtered out to give oseltamivir phosphate in 75% yield (0.2mol, 82g).
A second crop of presumably lower purity crystals can be obtained by concentrated the solution and collecting a second crop of product.
References and futher reading:
Direct conversion of ethers to esters by trichloroisocyanuric acid, tetrahedron letters, no 55, pg 5819-5820 (easy prep of ethyl acetate from ether,
pdf was found on SM)
See the threads here for stuff on thionyl chloride, especially thionyl chloride from sulfur dichloride and SO3 (and see the thread on that too),
acetic anhydride, dimethylformamide (dimethylacetamide is made in pretty much the same procedure but with acetic acid vs. formic acid),
Short and Efficient synthesis of optically active N-tosyl aziridines from 2-amino alchols, Lother W. Bieber & Maria C.F. da Araujo, Molecules
2002, 7, 902-906 (found on the net somewhere)
Reduction of azides to amines or amides with zinv and ammonium chloride as a reducing agent, W. Lin, X. Zhang, Z. He, Y. Jin, L. Gong, Synth. Commun.
32(21), 3279-3284 (2002) (found the essential details from here on a rhodium backup)
Preparation of Methanesulfonyl chloride (see the thread and attached pdf for the synth) in this case applied to making toluenesulfonyl chloride since
that is OTC
See US Patent 4,725,311 for details on making easy sodium in decent amounts OTC
|
|
|
Esplosivo
Hazard to Others
  
Posts: 491
Registered: 7-2-2004
Location: Mediterranean
Member Is Offline
Mood: Quantized
|
|
Nice work here  Pitty I can't get my hands on some of the chemicals mentioned in
the synthetic pathway. I will try getting some azides from air-bag systems and buy another couple of chemicals. In my country we are to be given free
neuramidase inhibitor (Tamiflu) if one's doctor prescribes it after reporting sickness to the authority. It would still be great to be able to carry
out the synthesis and who knows, if all hell breaks loose, it might turn out useful afterall. The supply is unfortunately not that large, where 25% of
the population is covered, although I do understand the procedure. Pitty I can't get my hands on some of the chemicals mentioned in
the synthetic pathway. I will try getting some azides from air-bag systems and buy another couple of chemicals. In my country we are to be given free
neuramidase inhibitor (Tamiflu) if one's doctor prescribes it after reporting sickness to the authority. It would still be great to be able to carry
out the synthesis and who knows, if all hell breaks loose, it might turn out useful afterall. The supply is unfortunately not that large, where 25% of
the population is covered, although I do understand the procedure.
Anyways, let us focus on the neuraminidase inhibitors. There are two other commonly known antiviral drugs, Amatadine and Zanamivir. The former,
Amatadine, turns out to be pretty much uneffective, since it is only effective against Influenza A and the influenza is quick to mutate and become
resistant to it, besides some other side effects which do not make it feasable. A report (the NICE report if IIRC) has shown that it does not show any
correlation with the reduction in the number of hospitalizations and may lead to other complications. Therefore synthesizing it would be a waste of
time and resources.
Zamavir on the other hand seems good enough, being able to treat Influenza A and B. It is contraindicated in children smaller than 12 years and in
people with asthma or Chronic Pulmonary Disease because it seems to be able to cause bronchospasm. Zamavir is taken as an inhaler, and that is a
disadvantage if one intends to produce it OTC.
Thus, Ostelamivir seems to be the best solution. Obviously I would recommend a normal seasonal influenza vaccine. Risking it won't cost anything (or
pretty much nothing) and the pandemic influenza might have certain epitopes (resulting from 'fusion' of the avian and seasonal influenza -
hybridization) which the bopdy might be primed to recognize by the vaccine and thus one will have a larger and faster immune response to the pandemic.
Oseltamivir prophylaxis has been shown to very effective in reducing the occurence of influenza. Many countries are stockpiling neuramidases for
prophylactic treatment of front-line workers (doctors, nurses, surgeons, etc...). Thus in many countries Oseltamivir are being used mainly as a
treatment following infection (recommended before 24-48hours max. following infection). Hope this helps. Ask if you need any other info.
Edit: The synthetic pathway of oseltamivir may be found on wikipedia - link. It may be useful for you to check out certain steps in the synthesis.
[Edited on 23-1-2006 by Esplosivo]
Theory guides, experiment decides.
|
|
|
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
Yes, I have seen the pathway detailed on wikipedia before, and have the actual journal with all the details for it. But, for the extra amount of
non-easily OTCable chemicals needed for a 1 to 2% increase in total yeild, I decided to stick with the original azide route since the chemicals are
cheap and the procedures are easy. Avoiding azides makes sense ofr a 100kg batch but none of us here are going to be doing those. I am thinking 100g
batches would be the most you could do easily on a large lab scale if you managed to do everything before on a larger scale.
What are the chemicals in particular you are not able to get. Everything in there is OTC or can be made from OTC starting materials. I am also
planning on the airbag route for azides since production on this scale will pose problematic.
Two other neuraminidase inhibitors, both orally available and more active than tamiflu, are "RWJ 207201" and "A 315675". Both have not been released
commercially. I have found a patent for A 315675 that might be OTC-able but it looks an order of magnitude more difficult and lower yielding
than oseltamivir. The patent in question is #6,455,571... though it took me half an hour to find what reaction scheme produced it (it is structure
50a) and I still haven't figured out what examples detail the reactions.
I have attached structural diagrams of the two drugs.
Another disadvantage with Zanamivir is that administration is difficult when trying to use it against a virus that attacks the lungs. It is more
active than oseltamivir, but its poor bioavailability takes away that advantage. The above two compounds have good bioavailability and are also more
active than zanamivir. A315675 in particular is a very strong inhibitor, and binds very strongly to nueraminidase. While oseltamivir has a half-life
of about 33 minutes, A315675 has a half life of 12 hours! (Note: these values are not representative of in body conditions, and represent an ideal
situation for the de-bonding of the drugs to the neuraminidase) It also seems to average out to be roughly 10 times more active.
But, as I said before, I haven't worked out the procedure yet, but it does not look OTC without vast amounts of work, and even then it looks like a
poor yielding procedure. I would much rather focus my lab resources towards something I am sure I can make than towards something I might be
able to make.
However, if an oseltamivir resitant strian goes pandemic, looks like at least knowing a procedure would be useful since it would them be pretty much
your only hope.
[Edited on 24-1-2006 by Chris The Great]
|
|
|
Pommie
Hazard to Self
 
Posts: 70
Registered: 6-2-2005
Location: Australia
Member Is Offline
Mood: No Mood
|
|
As a muggle, I was intrigued to see this appear on this screen. As a muggle with family I was also excited at the possibility that with some hard
study and some yet to learn chemistry that I may get access to the holy grail that Tamiflu will become if bird flu goes world wide.
Well, after nearly a month, nobody else has shown the slightest interest.
Is this because:-
The above synthesis is bobbins (Yorkshire term for garbage)?
Bird flu wont happen?
Your all talking quietly between yourselves?
It's all too hard?
Some other reason?
Sorry to bring this up, but it really does seem like a good idea.
Come on you knowledgeable people.
Mike.
P.S. muggle = thicky.
P.P.S. If this is doable, then I will have lot’s more questions.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
It's firstly because the synthesis will never be doable with OTC materials. NEVER. Period.
And even when the important non-OTC reagents (furan, THF, triethylamine, sodium, thionyl chloride and so on) are bought from a supplier or the
pharmacy, which is crucial for having the chance to make it, the synthesis will still be very long and have many pitfalls that are not listed here.
You can't expect every step to succeed the first time, and you can't expect to get near the yields given in literature.
Secondly, it's becaue the bird flu issue is massively inflated by the media, and there's actually no real threat to anyone living in a country where
people don't have daily body contact with birds or living chicken.
I won't even begin to describe how ordinary illnesses are so much more dangerous than the bird flu.
|
|
|
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
Oh, but it is OTC. Let me describe briefly. I have been quite busy so no fancy writeup.
Methyl acrylate:
glycerin, heat with magnesium sulfate-> acrolien, oxydise with O2 and reflux, add methanol and H2SO4 to get methyl acrylate.
Furan:
Distill wood dust, corn cobs, oats, etc with dilute hydrochloric acid (some sulfuric acid helps as well) to get furfural. React with sodium hydroxide
to get sodium furancarboxylate and furfural alcohol. Distill out the alcohol, acidify the remainder, and heat to decomposition. Furan distills out.
THF:
Raney nickel hydrogenation of furan.
Triethylamine:
Ethanol, sulfuric and NaBr are refluxed to give ethyl iodide. React with sodium cyanamide to get diethylamine (I posted a synth in the novocaine
thread). Mix together with more ethyl bromide (1:1 molar quantities) and reflux or heat under pressure, to get triethylamine bromide. NaOH and
distill to get triethylamine.
Sodium:
Reduction of sodium hydroxide with magnesium in refluxing kerosene in oxygen free atmosphere. (Bromic has been doing work on this recently).
Thionyl chloride:
Heat sodium bisulfate to red heat to distill out SO3. Make SCl2 (discussed on this board) from sulfur and chlorine. React the two to get thionyl
chloride.
Ethyl acetate:
Stir diethylether with TCCA overnight.
Diethyl ether is from ethyl alcohol and sulfuric acid, plus heat.
3-pentanol:
Acrolien is reduced to 1-propanol. This is oxydized to 1-propanal (aldehyde).
Ethyl bromide is reacted with magnesium (or lithium) to for ethylmagnesium bromide or ethyllithium. This reacts with the 1-propanal to form lithium
pentnoxide, which gives 3-pentanol on reaction with water.
Acetonitrile:
Acetic acid nuetralizes ammonia to form ammonium acetate, which gives acetamide on strong heating. This is dehydrated with thionyl chloride to for
acetonitrile.
Acetic anhydride:
Acetic acid + thionyl chloride to get acetyl chloride (might work in the synth instead of anhydride). React with sodium acetate to get acetic
anhydride.
Tosyl chloride:
Distill toluene and sulfuric acid to get toluenesulphonic acid (toluene removes water by forming an azeotrope). React with thionyl chloride to get
tosyl chloride.
Dimethylacetamide:
Formaldehyde and ammonium chloride react to form dimethylamine hydrochloride under reflux. The free amine is released with NaOH and neutralized with
acetic acid. The dimethylammonium acetate is heated to convert it to dimethylacetamide.
Sodium azide:
Get it from airbags. If not, make hydrazine with ammonia or urea reacting with highly basic hypochlorite solution. Add sulfuric to ppt. the sulfate.
Reduce potassium nitrate to nitrite using lead/carbon/whatever (see threads on this), make ethyl nitrite by reacting it with ethanol and H2SO4.
React hydrazine sulfate with NaOH to give hydrazine, react with ethyl nitrite and sodium hydroxide to get sodium azide.
Details are available here, orgsyn.org , and especially in archives of sites like rhodium and the hive.
Now, yes, it is very difficult. It will not get yields that high. It will not be success on every try. But, it is very possible, if you are
determined enough and have the time.
Nobody is afraid of avain flu in its current form. People are afraid because as an influenza virus, it is likely to mutate to be able to spread
easily between humans, as easily as the flu, but far more lethal. It has already mutated closer to that, as evidenced by the large infected family
clusters appearing with every new case.
So yes, currently, bird flu is quite safe and harmless. But 1918 spanish flu was harmless as well, until one day inside a pig it mutated to spread
amoung humans. Suddenly, 6 weeks later with slow 1918 travel, no massive crowds or airtravel worldworld, it has spread worldwide. Almost every
community is hit and over 50 million die. H5N1 doesn't even need to get in a pig, it can spread directly from birds to humans. That is one less step
it needs to mutate and spread. SARS showed us that it takes no time at all for an infectious disease to spread worlwide. And H5N1 looks more deadly
than its 1918 friend. Our entire society is dependant on constant worldwide movement of goods, without that we will have no food, water etc
avalaible.
And look what happens (New Orleans) when the safety net of society is taken away.
|
|
|
Pommie
Hazard to Self
 
Posts: 70
Registered: 6-2-2005
Location: Australia
Member Is Offline
Mood: No Mood
|
|
Well, just got in from the pub. So, thought I'd play devils advocate.
Is this doable?
I got as far as Raney Nckel Hdrogenation and realised its well beyond my abiliteis ( and my spealing)
But, the question remains, can it be done?
Mike.
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
The synthesis has 13 steps. If the yield is 75% at each stage (which would be good going for a lot of reactions) then the overall yield would be
0.75^13 ie about 1/40 so for each gram of product you would need about 40g of starting material (very roughly). That's probably managable.
If the yield is 50% which might be a better assumption then you get 0.5^13 ie about 1/8000 ie you need 8KG of stuff to get a gram of product- of
course, you also need the equipment to do the reactions.
If you have to make the starting materials by an array of another 12 syntheses then I think its fair to say that it's impractical even if it's
possible.
Breaking into a pharmacy looks like the easy way to get this stuff.
Anyway, if this 'flu takes hold then anyone with cash is going to want the drugs. At that point a lot of commercial labs will start making and selling
these compounds (and worrying about paying royalties to patent holders later. The Govt may well overrule the patent laws as an emergency measure).
I'm not at all happy about the idea of a pandemic but, can I remind you of something? You are all decended from people who survived the 1918 event.
You already have about as good a set of genes as you could hope for to survive this (fairly) similar pandemic.
Estimates of the death toll vary but here in the UK the highest estimate is about 500000 deaths. That's a lot, but the typical death toll for the UK
over the course of a year is about 750000 (about 60 000 000 folks in the UK of whom pretty much all will be dead in 80 years- a very crude estimate).
The worst case is, judged over a year, less than a doubling of the number of people who would have died anyway. Not nice, but not the end of the
world. OK, many of the dead will be the young adults so it will be disproportionately bad for the ecconomy but the species will survive and,
statistically, so will you.
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Sorry Chris The Great, but the notion to make this compound at home from OTC reagents is just redicilous. Not even with Corey's recent very efficient
and cheap pathway to Oseltamivir starting from 1,3-butadiene and acrylic acid would this be realistic.
In case someone is interested in details:
http://dx.doi.org/10.1021/ja0616433
Experimental:
http://pubs.acs.org/subscribe/journals/jacsat/suppinfo/ja061...
Fulltext: http://rapidshare.de/files/21628296/Oseltamivir.pdf.html
Shibasaki's route is another rescent one, it was actually published on the same day as Corey's:
http://dx.doi.org/10.1021/ja061696k
Experimental:
http://pubs3.acs.org/acs/journals/supporting_information.pag...
Fulltext: http://rapidshare.de/files/21628929/Shibasaki.pdf.html
He applies TMSN3, which is nothing you want to use on industrial scale, and still he unlike Corey chose to patent this inferior route.
|
|
|
azaleaemerson
Harmless

Posts: 27
Registered: 8-4-2005
Location: AR
Member Is Offline
Mood: No Mood
|
|
Acetic anhydride:
Acetic acid + thionyl chloride to get acetyl chloride (might work in the synth instead of anhydride). React with sodium acetate to get acetic
anhydride.
See step D above too.
Could the reason for AA versus AcCl be to have HOAc versus HCl as the byproduct? The milder acid by-product is less likely to chew on the parent
molecule?
Just thinking out loud
Azalea
azalea
|
|
|
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
It can be done with AcCl but the procedure is slightly different. I'd prefer it over AA as the yield is a bit higher for that step than with the AA.
I honestly see no reason other than low yields and large amount of work as to why this couldn't be done at home. I will continue in my (maybe
delusional) thinking until I have tried it and failed miserably.
I have a question about solvents though. In a lot of steps they use polar solvents, and I wonder if they could be replaced in many cases. For
example, I know dimethoxyethane is easily doable OTC and is very polar, could it for example replace dimethylacetamide or furan? Perhaps acetonitrile
as well?
Second question: Has anyone seen a good method of replacing NH2 with NH-C(=NH)-NH2 (guanyl)? I have only seen one method, it involves some
weird chemicals...
The reason being that if a good method is available, the tamiflu can be modified to have increased potency with just an isopropyl group, rather than
3-pentanol which will save a lot of large-scale grignards, if the NH2 is replaced with a guanyl group. Basically, the guanyl group increases the
potency, with little regard to the alkyl group at that location.
Thank you for those journals Sandmeyer, some of those reactions might come in handy at modifying my current chosen pathway to become more efficient.
Unionised, I could break into a pharmacy, but what the hell fun is that?
I figure that even getting a quarter the yeild I expect, it's 250 pills worth. With half of the expected yeild, 500 pills. By incorporating
probenecid into the pills, the oseltamivir is eleminated at half the rate, and so that doubles to 500 or 1000 pills.
Either way, a shitty yeild is still going to give hundreds of pills worth of product. If the guanyl group works, double those numbers again to 1000
to 2000 pills. 4000 for the expected yield (which will not be attained).
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
Before you buy a pill-pressing machine for this compound  , projects looking smooth
on paper pose unpredictible difficulties in the real life situation (lab), esp if the lab is a kitchen. What first seemed to be over in an afternoon
can turn out to be very time consuming even in a real lab and sometimes it simply dosen't work even if you follow allready published method exactly.
Don't think it's a good idea to plan in detail as all kind of weird things happen on the way... , projects looking smooth
on paper pose unpredictible difficulties in the real life situation (lab), esp if the lab is a kitchen. What first seemed to be over in an afternoon
can turn out to be very time consuming even in a real lab and sometimes it simply dosen't work even if you follow allready published method exactly.
Don't think it's a good idea to plan in detail as all kind of weird things happen on the way...
[Edited on 1-7-2006 by Sandmeyer]
|
|
|
organometallic
Hazard to Self
 
Posts: 53
Registered: 22-7-2007
Location: United Kingdom
Member Is Offline
Mood: No Mood
|
|
Guys, hes spent time on this theoretically sound synthesis, I for one reckon we should stop bashing him. Good work Chris, and a nice refresher on some
OTC preparations (sodium metal, need to read up on that..)
In vials of ivory and coloured glass
Unstoppered, lurked her strange synthetic perfumes,
Unguent, powdered, or liquid - troubled, confused
And drowned the sense in odours.
|
|
|
organometallic
Hazard to Self
 
Posts: 53
Registered: 22-7-2007
Location: United Kingdom
Member Is Offline
Mood: No Mood
|
|
ALso, chris, you prob want to start from shikimic acid, distilled from oil of star anise. Might be easier.
In vials of ivory and coloured glass
Unstoppered, lurked her strange synthetic perfumes,
Unguent, powdered, or liquid - troubled, confused
And drowned the sense in odours.
|
|
|
|