Catalysed
Harmless

Posts: 2
Registered: 4-8-2021
Member Is Offline
|
|
Synthesis of benzonitrile using ammonium sulphamate and benzamide
Hi ScienceMadness!
I've recently preformed and uploaded a video on the preparation of Benzonitrile from benzamide and ammonium sulphamate in 66% yield which you can find
here: https://www.youtube.com/watch?v=WTFM_UNeyTg&t=7s&ab_...
I originally got the inspiration for this reaction from the sciencemadness forum but I could find any write-up / first hand experiences (if there is
kindly let me know and let me know you experience). I believe this method is far more accessible than that of using dehydrating agents such as P2O5 /
SOCl2 and also avoids toxic cyanide salts.
I'd love to hear any feedback you guys may have on the video and the reaction itself as ultimately I'm making these for the all us amateur / home
chemists out there  . .
Attachment: Reactions of Ammonium Sulphamate with Amides & Ureas.pdf (358kB)
This file has been downloaded 351 times
Attachment: synthesis-of-nitriles-by-fusion-of-amides-with-ammonium-sulphama-1950.pdf (284kB)
This file has been downloaded 343 times
|
|
|
Pumukli
National Hazard
   
Posts: 708
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Congratulations for the synthesis and the video!
As one who advocates this method for preparing nitriles am very pleased to see that someone actually tried it and achieved good results.
I tried it on nicotinamide but your results seem far better than mine.
Some sort of temperature measurement of the reaction mixture would have been nice! Especially because the reaction goes in steps at different
temperatures and a temperature vs. time curve would be really helpful for those who would like to follow your steps!
Some sort of characterization of the product would have also been nice! (Melting point, boiling point, tlc spots, )
The steam distillation step was interesting too. How much water vs. how many ml/g product?
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
Well i thought it was pretty good, that being said this was the first youtube chemistry instructional i have ever viewed, so my opinion is extremely
uninformed. I hope it helps you that i subscribed to your channel. Good luck, how many iterations did it take for you to settle on this method?
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Thats very nice!
I recently made benzonitrile directly from benzaldehyde and hydroxylammonium chloride though, in hot DMSO, I think thats even more accessible.
It worked also great on piperonal to the tongue-breaker piperonylinonitrile.
This was the method, of course only done halfway, and the nitrile precipitated with water from the DMSO: https://www.tandfonline.com/doi/abs/10.1080/0039791090321944...
Ah wait, I simply add my writeup from lately about the piperinylinonitrile in here too: | Quote: | -4,8g hydroxylammonium chloride, crudely crushed and then dissolved under warming and stirring in -10ml DMSO, and them given to, the beaker rinsed
too, a flask containing
-8,1g of piperonal(56mmol) in 5ml DMSO.
Kept this at an internal temperature of 85-90°C under stirring, then left it to cool to RT. and later added 150ml of water under stirring.
Later cooled the mix in the freezer for a short while, the precipitate got filtered, 2x20ml h2o washes were done and it was dried with moderate
heating.
The crude crystals, having a stench composed of mostly dimethyl sulfide with some piperonal note, and weighed 7,63g(92,6%, and very likely free from
MSM.
Etc, some recrystallisation attempts with 1-propanol instead of ethanol, and I lost some during that, but the crude nitrile was likely already good to
use after a wash and prolonged drying....
After the recrystallisation with aqueous propanol it was drying, and then weighed 6,48g, 44mmol, which corresponds to 78,6% of the theory.
Checked the melting point, it melted sharply at 90-91°C, which would be consisting with the literature , 91-93°C.
|
This is really great and practical as it can get, as it both forms the oxime and dehydrates it in-situ to the nitrile.
And if you wish to, you could even turn that nitrile in-situ into an amide... probably also into an acid, or with slightly more effort, into an amine.
|
|
|
Catalysed
Harmless

Posts: 2
Registered: 4-8-2021
Member Is Offline
|
|
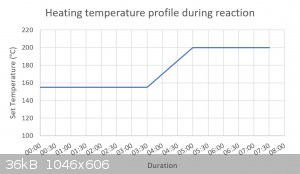
Thanks for the ideas Pumukil, measuring the reaction temperature would be fantastic although my two-necked 500ml RBF doesn't fit into my heating
mantle, awkward. The best I can do for now at least is go purely on the temperature settings on my mantle. I created a quick graph showing the heating
profile.
Characterization is also something I really did over looked, not the correct way to do chemistry whatsoever but an immiscible liquid with a
characteristic smell and density ~1g/ml made me sure I made benzonitrile. I'll really keep that in mind in future videos.
@Panache, I actually just performed this reaction twice, first time I thought it didn't work and stopped filming after seeing nothing in the
collecting flask. Only until I added water and saw some oily droplets did I realize it worked. In the end, I repeated the same reaction once more at
the same conditions. Benzonitrile wasn't exactly a goal of mine by itself but instead , the goal was directed at exploring and testing the sulfamate
dehydration reaction as its quite convenient and amateur friendly. There exist plenty of optimizing opportunities.
@Karlos, I don't think you will believe me but I recently began testing this reaction too! I've decided to use vanillin as my aldehyde, since its
accessible and stable. What further chemistry would piperonylonitrile bring? Great to see you have had such a great success with the reaction!
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Quote: Originally posted by Catalysed  |
@Karlos, I don't think you will believe me but I recently began testing this reaction too! I've decided to use vanillin as my aldehyde, since its
accessible and stable. What further chemistry would piperonylonitrile bring? Great to see you have had such a great success with the reaction!
|
Thats a cool coincidence! 
I plan to run a grignard on it, making "propiopiperone" as I like to call the 3,4-methylenedioxypropiophenone.
Just need to dissolve some copper in HI first for the copper(I) iodide.
I haven't calculated how much they actually use in the attached paper, like a few percent molar, but I know that without this, the yield would be like
a third of that at most.
I want to try the same with a longer grignard reagent, pentylmagnesium bromide, for hexanophenone on the benzonitrile.
|
|
|
|