chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
Bromopropiophenone using GAA
Dumb question. Can propiophenone be brominated in GAA in the same way it can be in DCM?
How much GAA should I use per ml of Propiophenone? propiophenone is really hard to get in my area so I dont wanna mess this up to bad and have to get
more. Just wondering if anyone who’s done it knows anything. I saw post from a while ago about it but it was never confirmed.
|
|
|
dextro88
Hazard to Self
 
Posts: 60
Registered: 20-10-2018
Location: In the lab
Member Is Offline
Mood: loading...
|
|
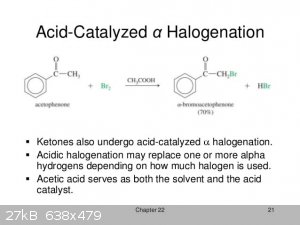
You might get some aromatic bromination wtih GAA as solvents espesialy when temperatures rise, i woud better use the good old DCM for this job, and
you can easily remove it afther the bromination and all its traces, otherwise the neutralization in GAA is nasty, and in my little chemistry
experience thats a good solvent for aromatics rings halogenations.
[Edited on 23-8-2021 by dextro88]
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
....there was an interesting discussion on this subject some time ago..which may shed some light in addition to current topic....solo
https://the-hive.archive.erowid.org/forum/showflat.pl?Number...
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
You mean if you can use another solvent instead?
Of course!
You can even use other and better brominating agents, much less hassle and no annoying work with liquids at all besides your soon-to-be-bromoket
I used the method from the attachment to prepare a quite large number of aminoketones, and especially the variant without a solvent, using 10 molar %
of the NBS in tosic acid as catalyst, carefully and slowly allowing it until molten at the start, lots of room overhead, will after a little bit of
practical experience will result in mostly quantitative yields, yes, probably only very few exceptions.
In the start I had a few due to it, but all of them could be resolved and now I get regularly, depending on the ketone, up to quantitaive
yields(especially with such aromatic C2 ketones like acetophenone and 2-acetonaphthone).
Desoxybenzoin is a bit lower yielding on desyl bromide, but I guess that has steric reasons and can be improved lke everything else.
Go try it, ideally buy directly a pound of NBS, it is such a great and valuable reagent that I can only recommend having some on stock at any point.
Be it oxidation, bromination, and so many cool appications else.
If you don't to buy NBS, then save your entire stock of bromine and buy succinimide instead, and make some.
NBS is really such a convenient and nice reagent, yet still very much overlooked.
Not even our russian friends made such extensive use of NBS for their cathinone precursor preparation, and these substances are more common over
there, they still stick to bromine somehow.
Well... I've been told hmm.. around I would guess, maybe one and a half dozen times, from people experienced in these reactions, but with bromine,
that they will never go with bromine itself again if they can avoid it, and how this justifies going a way to make the NBS on your own, as it is just
so convenient.
(at this point, my mental image will usually nod approvingly and tick a mark in a box...)
Which is more or less exactly what I've been saying for years now, over and over, like a broken record  
Attachment: _05_BUPROPION_Synthetic Communications.pdf (247kB)
This file has been downloaded 449 times
|
|
|
theAngryLittleBunny
Hazard to Others
  
Posts: 130
Registered: 7-3-2017
Location: Austria
Member Is Offline
Mood: No Mood
|
|
Oh wow thanks! I always used bromine and and my yields were usually around like 30%. I was wondering where the other 70% went, but I never really
cared since these ketones were quite cheap for me, but that is really good to know. And since NBS has 90% the potency of pure bromine, you only need a
little bit more then with pure bromine.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Yeah well, bromine is fine and all, but besides the low yields(you only get those high ones with NBS with the right acid catalyst though!) is it not
even close as versatile as NBS.
You can even go straight from a 1-arylalkanol to the bromoketone of said ketone, or if you want to attach a secondary amine, one-pot all the way with
some indian method.
Countless other uses, but especially regarding aminoketones it is really useful.
I couldn't list them all if I wanted to; even though I spent half the last weekend to sort my files about the stuff.
verrückt und wissenschaftlich
|
|
|
theAngryLittleBunny
Hazard to Others
  
Posts: 130
Registered: 7-3-2017
Location: Austria
Member Is Offline
Mood: No Mood
|
|
Oh, so NBS also oxidizes secondary alcohols to ketones as well? That sounds cool, I always just thought NBS was used by people who just don't wanna
deal with bromine. It would also spare you the clouds of HBr coming out of the reaction mixture during bromination, which sounds nice.
But I'm not sure if that's a dumb question, but couldn't TCCA (Trichloroisocyanuric acid) theoretically also work sometimes in place of NBS to make
the chloroketone? I mean it technically has the same funcional groups as NBS. Maybe I could give that a try sometime.
Quote: Originally posted by chemist1243  | Dumb question. Can propiophenone be brominated in GAA in the same way it can be in DCM?
How much GAA should I use per ml of Propiophenone? propiophenone is really hard to get in my area so I dont wanna mess this up to bad and have to get
more. Just wondering if anyone who’s done it knows anything. I saw post from a while ago about it but it was never confirmed.
|
I always used toluene or hexanes as a solvent which worked for me. Only the first time I used DCM and I don't think there was a difference in my
yield. I just didn't wanna use DCM in the future because I don't think it's a good solvent for any reactions involving amines.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Well I wouldn't want to use the chloroketone... it is even a worse lachrymator than the bromoketone 
But that paper deals with the issue: https://doi.org/10.1080/00397918508063816
Yeah if you don't want to deal with bromine... and don't want to deal with miserable yields while at it too 
verrückt und wissenschaftlich
|
|
|
theAngryLittleBunny
Hazard to Others
  
Posts: 130
Registered: 7-3-2017
Location: Austria
Member Is Offline
Mood: No Mood
|
|
And thanks, bromine never really bothered me much, not nearly as much as the haloketones. But after reading that I could have spared myself making
bromine for this all along, annoying, but nice to know now.
I can't really imagine it makes much of a difference if it's the bromoketone or chloroketone, both are horrific, the chloroketone would just be a
little bit more volatile, and volatility isn't really a problem with stuff like bromopropiophenone. But what is horrific is getting a tiny bit of the
bromoketone on my finger without noticing and touching my face, then I had to spend the next 30 to 60 minutes scrubbing my face in the shower. I
learned my lesson and would always wear gloves after that while soaking putting anything that came in contact with it in a sodium hydroxide solution
as soon as possible.
[Edited on 12-9-2021 by theAngryLittleBunny]
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Ozone generator, works apparently well against such smells.
Not sure if sodium hydroxide solution does anything beneficial.
If you get this on you next time, do it different, like this: keep an old close, in case you got some on you wet the rag with ethanol and then you
wipe the respective spot off, carefully, not too strong, repeat this three times in total(with a different spot of the rag), wash them(=the hands of
course) another time in alcohol, this time properly as if washing with water, followed by water and soap.
If you use acetone, its more effective, but your skin gets more damaged and dried out, and it burns a hell lot more on damaged skin if you have rubbed
a spot too hard.
But this rubbing and alcohol method removes the bromoketone pretty well from the skin into the rag, and unlike water, does not cause it to precipitate
where it is, which is why washing it immediately with water off, does not much at all.
And then I would put some cream on it to take care of the skin, after all that defatting solvent on it.
Apparently, in russia a certain substituted propiophenone, or rather the bromoketone, controlled, it became effectively illegal to buy and sell.
Thing is, in russia, you could even buy kits to make drugs with, just pour the bottles in respective order together, wait the designated time, and
in-situ a white crystalline mass precipitates.
Filtered off. washed with the last bottle, and dried 
I have seen a video from such a thing, could figure out what it was made off(probably IPA solutions of methylamine and the haloketone poured together,
then HCl and acetone, making the salt and precipitating it, and another acetone wash).
Its a crazy sight for sure.
Well, what I wanted to say is, now with the bromoketone becoming restricted, they started to use the iodoketone instead.
It is also a lachrymator, but less strong as is less volatile of course.
I actually heard from somebody who dropped a flask with like a half liter of bromoketone in a bathroom of his house... said hundreds of liters of
acetone were used, its still noticeable and so on... horrible...
An ozone generator would have fixed this apparently in no time.
Also other solvent smells.
As for NBS, yeah its one of the advantages you don't need to make bromine, sure.
But, how much do you pay for bromine actually if you make it yourself?
Compare that with the price of a pound or kilo if NBS 
Its 5g each 25mmol of substrate, with 500g being sufficient for 2,5 mol of a propiophenone..
Its not that much but its effectivity makes up for that.
E: think got all the typos out 
[Edited on 12-9-2021 by karlos³]
verrückt und wissenschaftlich
|
|
|
theAngryLittleBunny
Hazard to Others
  
Posts: 130
Registered: 7-3-2017
Location: Austria
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by karlos³  |
I actually heard from somebody who dropped a flask with like a half liter of bromoketone in a bathroom of his house... said hundreds of liters of
acetone were used, its still noticeable and so on... horrible...
[Edited on 12-9-2021 by karlos³] |
HOLY SHIT! This kind of stuff happens in my worst nightmares, I spilled a few mL of a regular high boiling ketone a few days ago and that already
sucked. I'm not a the strictest person with safety if I'm honest, but I would never ever keep any haloketone around. I would even react it in the same
beaker I made it, let alone storing half a liter of this stuff, you could make a terrorist attack with that. Why couldn't they just put the regular
ketone with some NBS in that kit? But yeah, cathinones are something you could easily teach anyone to cook by following a recipe. And I would guess
the iodoketone would be several times as expensive to make though simply because of the price of iodine compared to bromine. If they keep restricting,
at some point there will be kits where you'd have to do the friedel craft acylation to make the ketone lol.
And NaOH destroys bromoketones quickly, it's called Favorskii rearrangement, in my experience it always destroyed any bromoketone residue. And I know
alcohol would work better then water, but when my face was burning finding a bottle of some alcohol or even thinking about that wasn't something I was
able to do. And of course I didn't think about that beforehand since I didn't know how much it would burn, but thank you for that tip.
And I meant that you could chlorinate the ketones with TCCA, NBS is kinda expensive compared to just making bromine, but TCCA is really cheap
obviously, so if that works I would only use that from now on lol.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
I think that guy was doing something more sinister with that much.
Personally, I prefer to bottle some up and when I brominate ketone, I alrady have a bottle ready where it goes in and thats very practical, this is so
much easier to simply make some, and whenever you need some, you measure a few ml's or what you desire out, no extra reaction, not twenty times
instead of just one time(ok, in obscene amounts...) for the damn confrontation with the bromoketone.
No, just once pull yourself together, whip up a half mole(that was almost the most I did, and consequentially, didn't needed to run another for over a
half year  ) and think of the time it saves you after you prepared a bottle full ) and think of the time it saves you after you prepared a bottle full

Check S3 for NBS, a half kilo is under 50 euros.
Thats cheap, compared to every other seller I ever looked for NBS, something outrageously expensive.
Not S3 though.
Well, would TCCA work? I believe yes, definitely.
But I think it will be far more unpleasant, a lot even.
verrückt und wissenschaftlich
|
|
|
theAngryLittleBunny
Hazard to Others
  
Posts: 130
Registered: 7-3-2017
Location: Austria
Member Is Offline
Mood: No Mood
|
|
Do you live in Europe? I used this S3 chemicals a lot, I think it's so weird they sell 4-Methylpropiophenone, like it's so obvious what many people
would use that for. And if I had to do it 20 times that was fine for me, later on I also only handled anything that would come into contact with the
bromoketone inside a plastic tub so I know don't forget what's conterminated with it. I had stuff like where I would accidentally touch a liquid and
then would smell my hand to see what it is and sometimes getting it a bit on my face (My coordination isn't the best) and then I would realize
"oh....that's benzyl bromide" because if would take a few seconds to burn. Benzyl halides and haloketones are one of a few things I just don't wanna
have around. Bromine is okay because when I would spill a bit on me it would be gone within a few seconds, and you know, haloketones tend to stick
around a bit longer.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
What, europe?
I had a more obvious mood last time, but this time.... if that would be in english, it would be "aminoketonologic", no?
Possibly we even speak a similar language, according to your country(note, I said similar  ). ).
verrückt und wissenschaftlich
|
|
|
|