Monoamine
Hazard to Others
  
Posts: 168
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Electrophilic chlorination
So I'm starting to draw up all the prerequisites for a planned electrophilic chlorination off an aromatic ring.
Here's the problem though: Unlike with some other Friedel Crafts type stuff, it's actually very difficult to control the amount of Cl2
introduced into the reaction, since it's pubbled in as a gas... and you also don't know exactly how much
So I was thinking about trying to control this a bit in the following way.
Since each substitution liberates HCl (check out this like for recao of the reaction mechanism, it may be possible to add the molar trimethylamine
into the mixture and, but add only as much trimethylamine as you want Cl's on your aromatic ring. Continuously monitor the PH of the reaction an when
it starts rising, then you know that the HCl sponge (trimethylamine) has been used up, and so now you can stop adding in more Cl2.
Of course, some extra Cl2 will still make its way to the the reaction cauldron, but if we add some water to the mixture, I think it will
destroy the anhydrous FeCl3 (or Al Cl3) thereby effectively brining the reaction to a halt and any remaining Clw
should react with the water to form more HCl. So at that point, you can basically just neutralize the remaining HCl by some mild base.
Note: To be honest I'm a bit worried about the step where water is added to the cauldron to destroy the anhydrous FeCl3,
since I'm not quite sure how violent this is... The reaction is as follows though:
FeCl3 + 2 H2 -> FeOCI(solid) + 2 HCl(gas).
But this would suggest that as long as you vent the HCl gas out and pass it through an alkaline solution you would be able to neutralize it too
(unless you want to keep it for something of course).
In fact, maybe this all unnecessary, and the HCl formed during the catalytic halogenation is also a gas (since the reaction is done in anhydrous
conditions, In which case scratch the pyridine and just put 1 molar equiv of NaOH in the HCl collection beaker solution and once its PH is neutral
then turn off the Cl2 generator.
Also, is there a general census as to what solvent this reaction should be done in? (assuming we're chlorinating something that's not a liquid already

I was going to try something like an alkane first, but if that's too nonpolar than maybe
I'll probably post a sketch of the setup in a bit, but I'm very curious to hear you insights, especially since I have a knack for overlooking many
things...
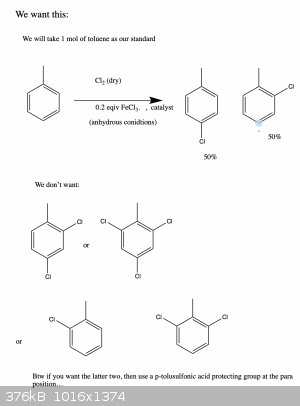
PS:An even more important question (and maybe a outside the scope of this post) are any of you aware of a protecting group that will protect
the para positions of a para-ortho director, but no the ortho position? So if you wanted to make para chloro toluene only, then you could do
this without doing the thing here and then having to somehow try to purify your products...
|
|
|
Oxy
Hazard to Others
  
Posts: 140
Registered: 1-12-2020
Member Is Offline
|
|
Quote: Originally posted by Monoamine  | So I'm starting to draw up all the prerequisites for a planned electrophilic chlorination off an aromatic ring.
Here's the problem though: Unlike with some other Friedel Crafts type stuff, it's actually very difficult to control the amount of Cl2
introduced into the reaction, since it's pubbled in as a gas... and you also don't know exactly how much
|
Halogens are deactivating substituents, not very strong but still. The product of multiple substitutions will be easy to separate by distillation as
there is significant boiling point difference
Quote: Originally posted by Monoamine  |
So I was thinking about trying to control this a bit in the following way.
Since each substitution liberates HCl (check out this like for recao of the reaction mechanism, it may be possible to add the molar trimethylamine
into the mixture and, but add only as much trimethylamine as you want Cl's on your aromatic ring. Continuously monitor the PH of the reaction an when
it starts rising, then you know that the HCl sponge (trimethylamine) has been used up, and so now you can stop adding in more Cl2.
Of course, some extra Cl2 will still make its way to the the reaction cauldron, but if we add some water to the mixture, I think it will
destroy the anhydrous FeCl3 (or Al Cl3) thereby effectively brining the reaction to a halt and any remaining Clw
should react with the water to form more HCl. So at that point, you can basically just neutralize the remaining HCl by some mild base.
|
Are you aware that you will mix Lewis acid and Lewis base? They will react very quickly and you will quickly loose all FeCl3 or other acid catalyst in
reaction mixture.
Quote: Originally posted by Monoamine  |
PS:An even more important question (and maybe a outside the scope of this post) are any of you aware of a protecting group that will protect
the para positions of a para-ortho director, but no the ortho position? So if you wanted to make para chloro toluene only, then you could do
this without doing the thing here and then having to somehow try to purify your products...
|
A convenient way of doing that would be defintely a sulfonation which is reversible and the compound can be easily desulfonated. Due to steric
hindrance the sulfonation will substitute in para position (if performed just once).
[Edited on 20-7-2021 by Oxy]
|
|
|
Monoamine
Hazard to Others
  
Posts: 168
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Possible recipe to do this:
Ok, so I thought about it a bit, and I think that it may be possible to do like this:
I tried to sketch the general setup for this:

Basically the general procedure would be:
Setup:
1) Set up a Cl2 generator (Lots of ways to do this, look on youtube).
2) Pass the Cl2 through conc. H2SO4 to dry it.
4) Place 1 mol of dry toluene together with 0.2 mol FeCl3 into a reaction vessel on a hotplate with magnetic stirring.
5) Pass the fumes from the reaction vessel through a solution containing 1 mol of dry triethylamine mixed with dry ether.
6) Finally, let the exhaust HCl and Cl2 fumes pass through water so you can recover some HCl (and a make a little HOCl), for later use (for instance
to generate Cl2). Or just neutralize it at that point if you don't want it anymore...
Procedure:
Basically, the whole point of this is to be able to control the amount of Cl2 that participates in the chlorination and to avoid
over-chlorinating the substrate.
1) Set up the apparatus as shown above.
2) Begin passing Cl2 through the reaction mixture.
3) So according to several places I looked this has to be done by adding "heat" (one of the most annoying terms in chemistry imo (come on we are
scientists we know how to quantify temperature numerically...) Since I wasn't able to find what temperature this so called `heat' is, I would just
start heating the reaction very slowly with stirring, until we can see the reaction begin. If anyone knows what temperature Friedel crafts
reactions are usually done at please let me know!
4) Recall that whenever we chlorinate toluene we produce one molecule of HCl (as a gas) which, then passes through the triethylamine + ether solution,
where it forms a salt (which is no soluble in ether and will therefore precipitate out of solution). This means that we know the reaction has started
when we see precipitate forming in bottle 4. (Note that Cl2 does not react with either of these solvents and just passes through.
5) Finally, any excell Cl2 and HCl will then be bubbled through a solution of distilled water where the we get
Cl2+H2O -> HCl + HOCl, allowing us to recover some HCl or simply neutralize the acidic fumes safely.
6) The reaction is done when the rate of triethylammonium hydroxide salt formation starts to get significantly slower.
7) At that point stop the Cl2 generation.
8) Remove vessel 4 and replace it with vessel 5.
9) To further quench the reaction, slowly add 0.3 mol of distilled H2O to the reaction vessel to destroy the FeCl3 via 2
FeCl3 + 3 H2O -> FeO3(solid) + 6 HCl(gas). The extra HCl will no go directly the the HCl
solution vessel 5.
10) At this point all that's left to do is is remove the solids in the reaction vessel by filtration and to subsequently
10) Finally, separate the two product isomers. The only think I can think of is using something like column chromatography, but if anyone else knows a
clever way then please let us all know!
Just as a side note: If you are generating chlorine at a very consistent rate every time then you might also be able do some experiments to see if
running the reaction for a certain time will give mostly single chlorinated products.
I might try this out one of these days (unless someone discovers a flaw which won't make it work - in which case please correct my
folly!).
Oh and again, if anyone knows of a protecting group which selectively protects the ortho positions of activators, I would be infinitely grateful to
learn about it, since I haven't been able to find one anywhere...
|
|
|
Monoamine
Hazard to Others
  
Posts: 168
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Quote: Originally posted by Monoamine  |
PS:An even more important question (and maybe a outside the scope of this post) are any of you aware of a protecting group that will protect
the para positions of a para-ortho director, but no the ortho position? So if you wanted to make para chloro toluene only, then you could do
this without doing the thing here and then having to somehow try to purify your products...
|
A convenient way of doing that would be defintely a sulfonation which is reversible and the compound can be easily desulfonated. Due to steric
hindrance the sulfonation will substitute in para position (if performed just once).
sulfonation is great for protecting the para position of para-ortho directors. What I mean was is there a protecting group with only protects the
ortho position so that you can only get the the para chloro product, for instance?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Snap back to reality: why are you doing this? Why control the amount of Cl2 introduced into the reaction? Usually, in order to obtain
monochlorotoluenes, toluene is used in excess. Usually, over-chlorination can be avoided by controlling the power of the chlorinating agent and the
heat of reaction. Chlorine doesn't sit around in the rxn solvent; it reacts or it boils away.
Anyway, you can't add trimethylamine or any other amine to a Friedel-Crafts, and the fact that you would suggest that makes me think you need to go
read more about what kind of role HCl plays as a "catalyst poison" in the Friedel-Crafts reaction (hint: it's not acting as an acid!).
|
|
|
Monoamine
Hazard to Others
  
Posts: 168
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Quote: Originally posted by clearly_not_atara  | Snap back to reality: why are you doing this? Why control the amount of Cl2 introduced into the reaction? Usually, in order to obtain
monochlorotoluenes, toluene is used in excess. Usually, over-chlorination can be avoided by controlling the power of the chlorinating agent and the
heat of reaction. Chlorine doesn't sit around in the rxn solvent; it reacts or it boils away.
Anyway, you can't add trimethylamine or any other amine to a Friedel-Crafts, and the fact that you would suggest that makes me think you need to go
read more about what kind of role HCl plays as a "catalyst poison" in the Friedel-Crafts reaction (hint: it's not acting as an acid!).
|
Let me try an address your points.
For the amine in the friedel crafts, if you look at the more detailed post I put up this afternoon, the idea was to have the trimethylamine in ether
after the Friedel crafts cauldron. That way excess Cl2 will just pass through, but the HCl will farm an insoluble salt whose
percipitate can be used to monitor the progress of the reaction. The role of triethylamine here is more as an indicator (since PH indicators are
difficult to use here because using them in something that provides protons would generate more HCl and give false results). It's not exact, by any
means, not least because some HCl will likely pass through without forming a salt, but maybe worth a try?
To answer you second question:
The reason I'm doing this is essentially for practice to get some hands on experience with electrophilic chlorinations. I'm later hoping to chlorinate
an benzene ring in a much larger structure, but only at one of the para positions (relative to the single bond at which the ring is connected to the
rest of the structure. I guess I could try to use twice the amount of protecting groups, but due to steriks I somehow doubt that I'll manage to get it
in....
Actually, let me explain this with a drawing and hopefully I'll make more sense:
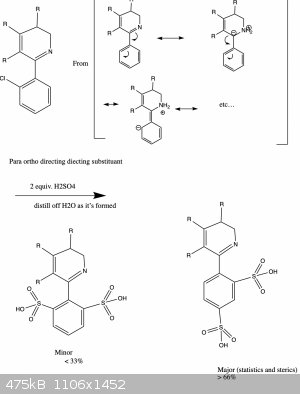
And now we're where we want to be!
But that still leaves your comment about amines (Lewis bases) and and Lewis acids... and that lone pair in that sp2 orbital is worrying me a bit...
I'll give it some thought.
Thanks for the comments my friend!
[Edited on 21-7-2021 by Monoamine]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I'm not convinced that aldimino/ketimino groups are ortho/para directing (or that they're compatible with FC rxn conditions in the first place!), and
your arrow-pushing diagram pulls two hydrogen atoms out of the aether, so those seem like more critical issues.
| Quote: | For the amine in the friedel crafts, if you look at the more detailed post I put up this afternoon, the idea was to have the trimethylamine in ether
after the Friedel crafts cauldron. That way excess Cl2 will just pass through, but the HCl will farm an insoluble salt whose percipitate can be used
to monitor the progress of the reaction. The role of triethylamine here is more as an indicator (since PH indicators are difficult to use here because
using them in something that provides protons would generate more HCl and give false results). It's not exact, by any means, not least because some
HCl will likely pass through without forming a salt, but maybe worth a try?
|
Are you aware that mixtures of ether and chlorine can explode? You can't just assume chlorine won't react
with stuff! It's very reactive!
https://drs.illinois.edu/Page/SafetyLibrary/ChemicalCompatib...
I'm not convinced that chlorine won't react with trimethylamine, either.
[Edited on 21-7-2021 by clearly_not_atara]
|
|
|
Monoamine
Hazard to Others
  
Posts: 168
Registered: 25-5-2021
Location: Sweden(ish)
Member Is Offline
Mood: +7
|
|
Quote: Originally posted by clearly_not_atara  | I'm not convinced that aldimino/ketimino groups are ortho/para directing (or that they're compatible with FC rxn conditions in the first place!), and
your arrow-pushing diagram pulls two hydrogen atoms out of the aether, so those seem like more critical issues.
| Quote: | For the amine in the friedel crafts, if you look at the more detailed post I put up this afternoon, the idea was to have the trimethylamine in ether
after the Friedel crafts cauldron. That way excess Cl2 will just pass through, but the HCl will farm an insoluble salt whose percipitate can be used
to monitor the progress of the reaction. The role of triethylamine here is more as an indicator (since PH indicators are difficult to use here because
using them in something that provides protons would generate more HCl and give false results). It's not exact, by any means, not least because some
HCl will likely pass through without forming a salt, but maybe worth a try?
|
Are you aware that mixtures of ether and chlorine can explode? You can't just assume chlorine won't react
with stuff! It's very reactive!
https://drs.illinois.edu/Page/SafetyLibrary/ChemicalCompatib...
I'm not convinced that chlorine won't react with trimethylamine, either.
[Edited on 21-7-2021 by clearly_not_atara] |
Yea the editor wouldn't let me delete them anymore so I tried to cover it with the charge lol.
"Are you aware that mixtures of ether and chlorine can explode? You can't just assume chlorine won't react with stuff!"
No! I did not know that! Thank you for the warning! So I guess it would have to be different non salt-dissolving solvent (reasonably
non polar), which is also aprotic and dissolves triethylamine...
Maybe a better solution would be do first pass the gasses through an alkene solvent so that the Cl2 will add across the pi bonds there and
get "soaked up" there? Then all that would be passing through the triethylamine bottle would be the HCl sin(I think). You'd also have to do some tests
to see how efficiently the Cl2 is trapped by this method or if some escapes and passes through anyway.
Never a dull moment in Science!
[Edited on 21-7-2021 by Monoamine]
|
|
|
|