Johnny Cappone
Hazard to Self
 
Posts: 74
Registered: 10-12-2020
Location: Brazil
Member Is Offline
|
|
"Instant Orange Juice" with Cuprous Hydroxide - Why is Cu(I) forming instead of Cu(II)?
Today I posted a video of the demonstration that I dubbed "instant orange juice with cuprous hydroxide" (https://youtu.be/UKUEsfNdRk8).
After discovering that the electrolysis of a concentrated NaCl solution with copper anode in a divided cell would produce CuCl and not CuCl2 as I
expected, I decided to take advantage of the unusual color of CuOH, in contrast to the other copper compounds, to try to produce some cool images.
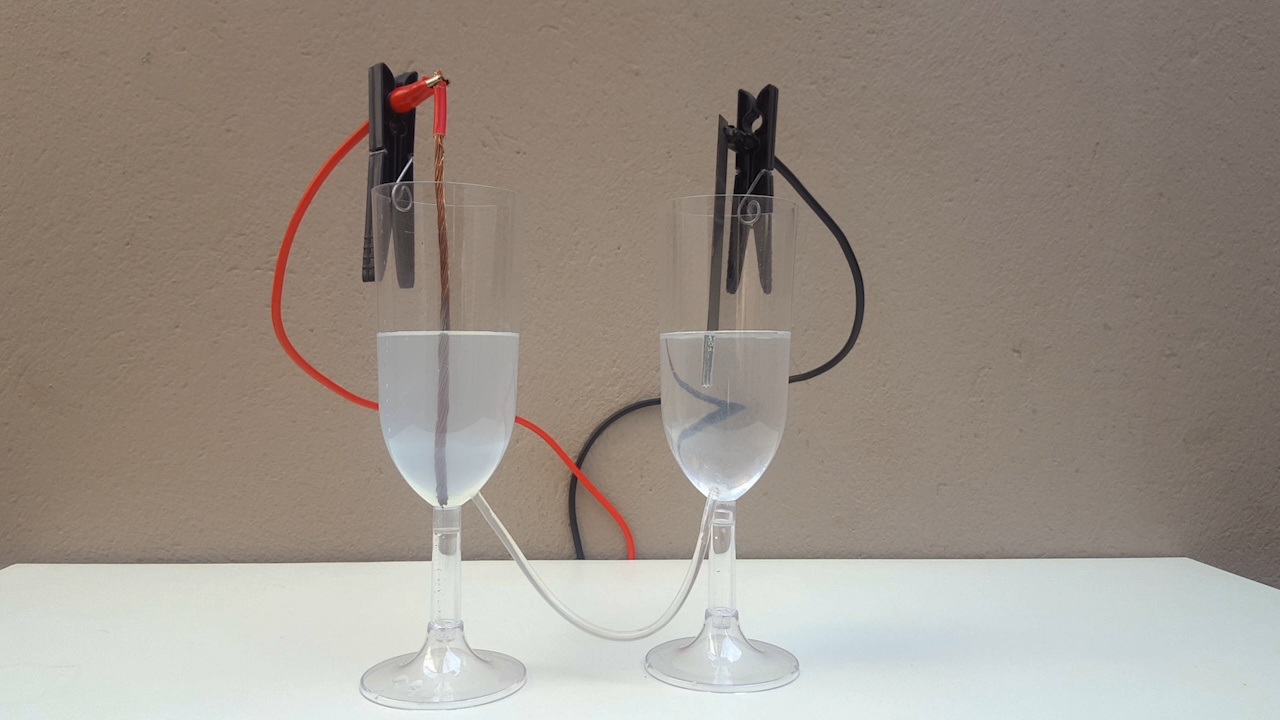
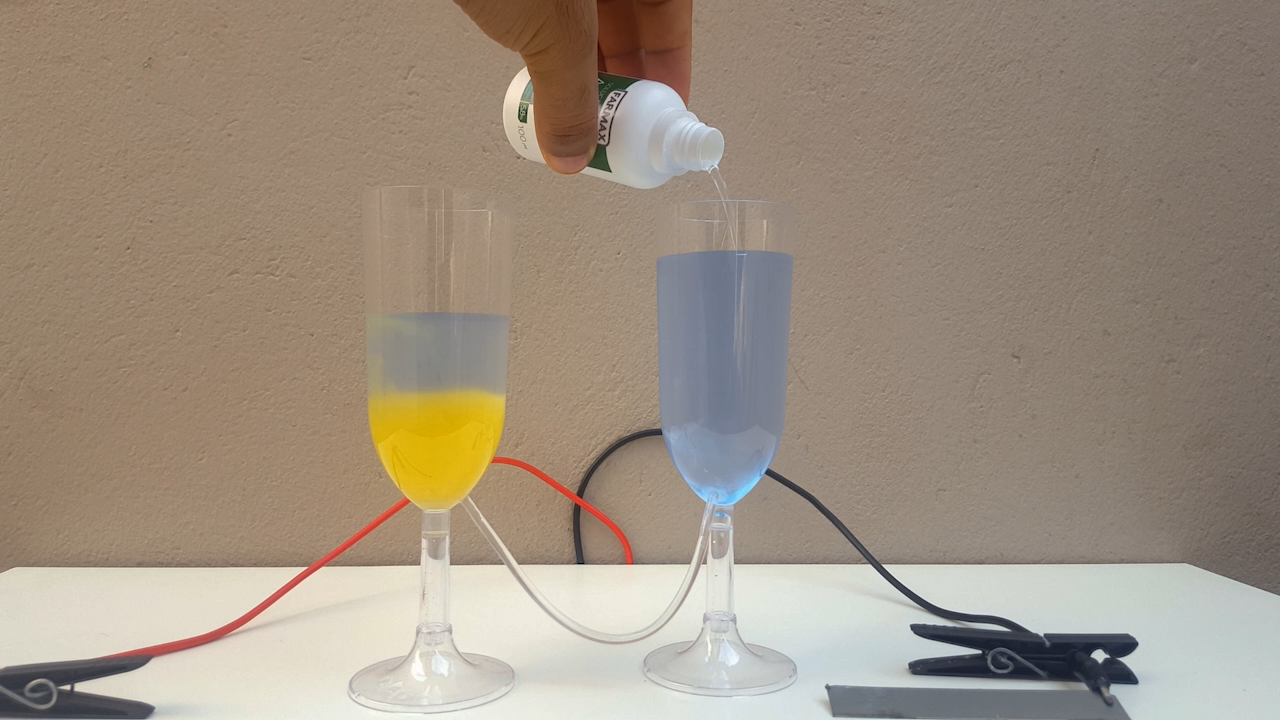
For this, I built a "cell" made up of two plastic cups joined by a thin transparent tube. In other words, a cheap (but functional) imitation of a
typical "U-tube" that you would find in any minimally equipped laboratory (photo 01)
The electrical resistivity obviously got a little high, it took 24 volts to make the electrolysis happen at a reasonable rate. Anyway, after some
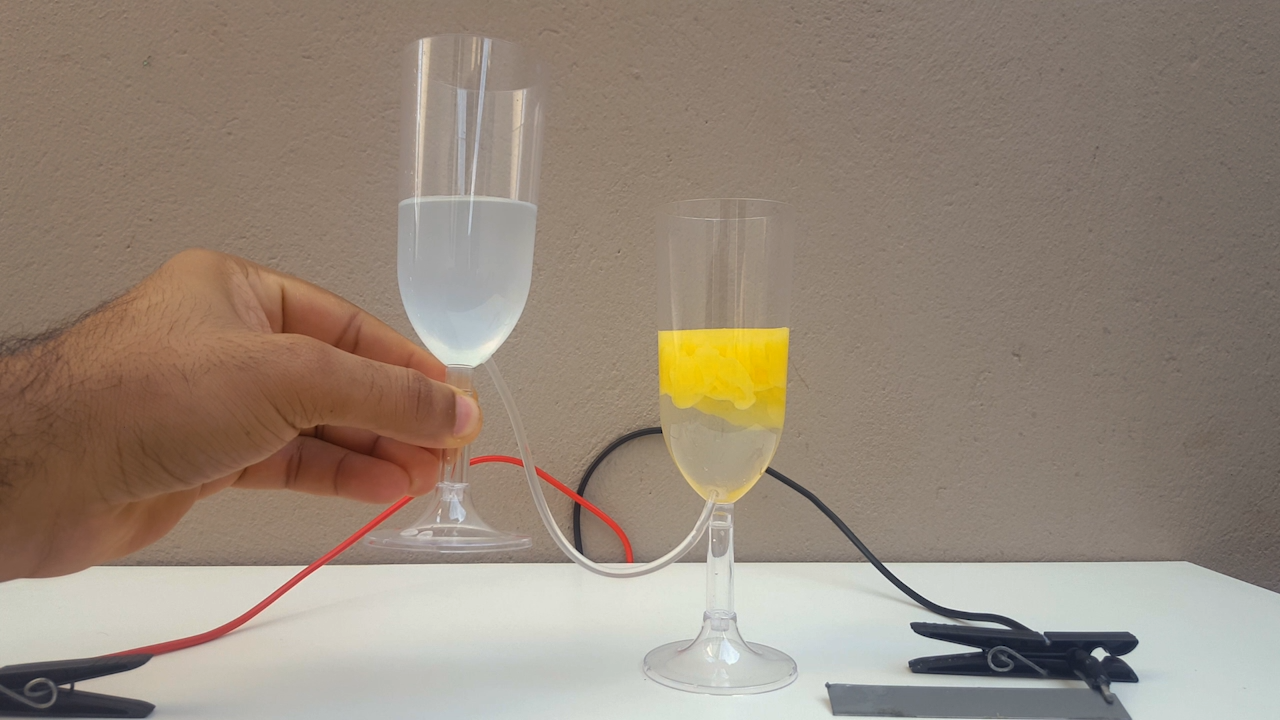
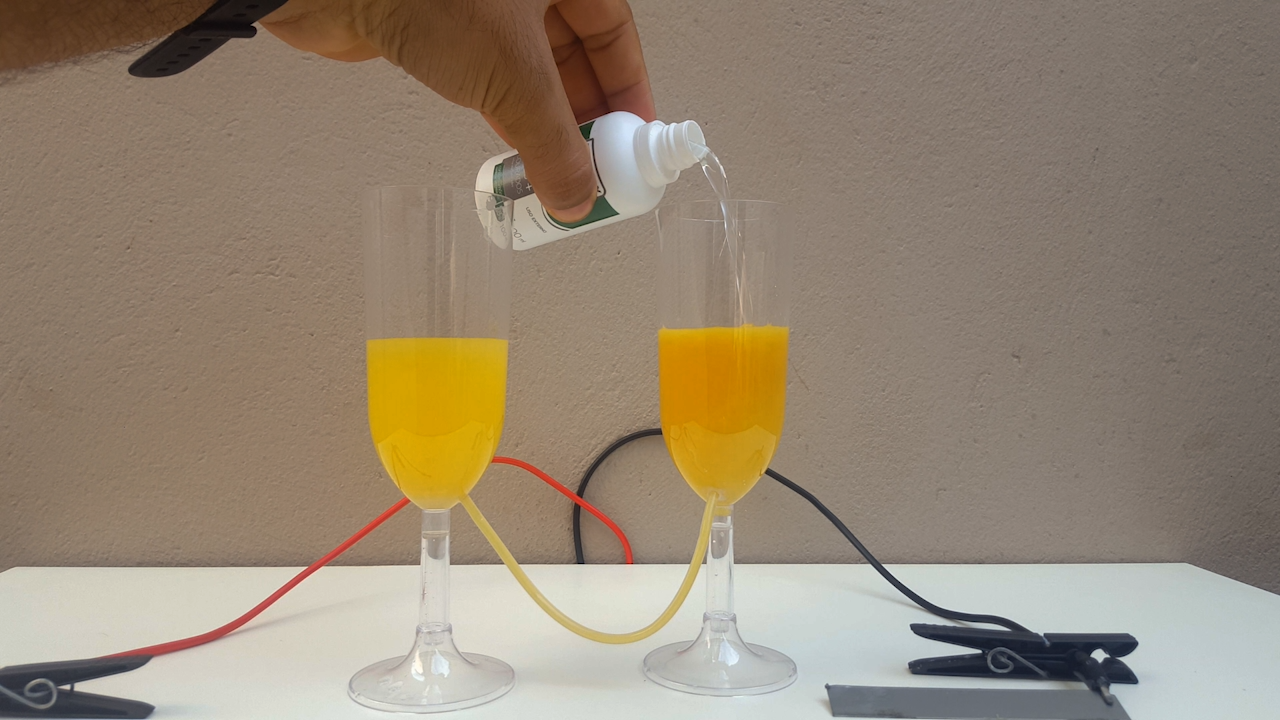
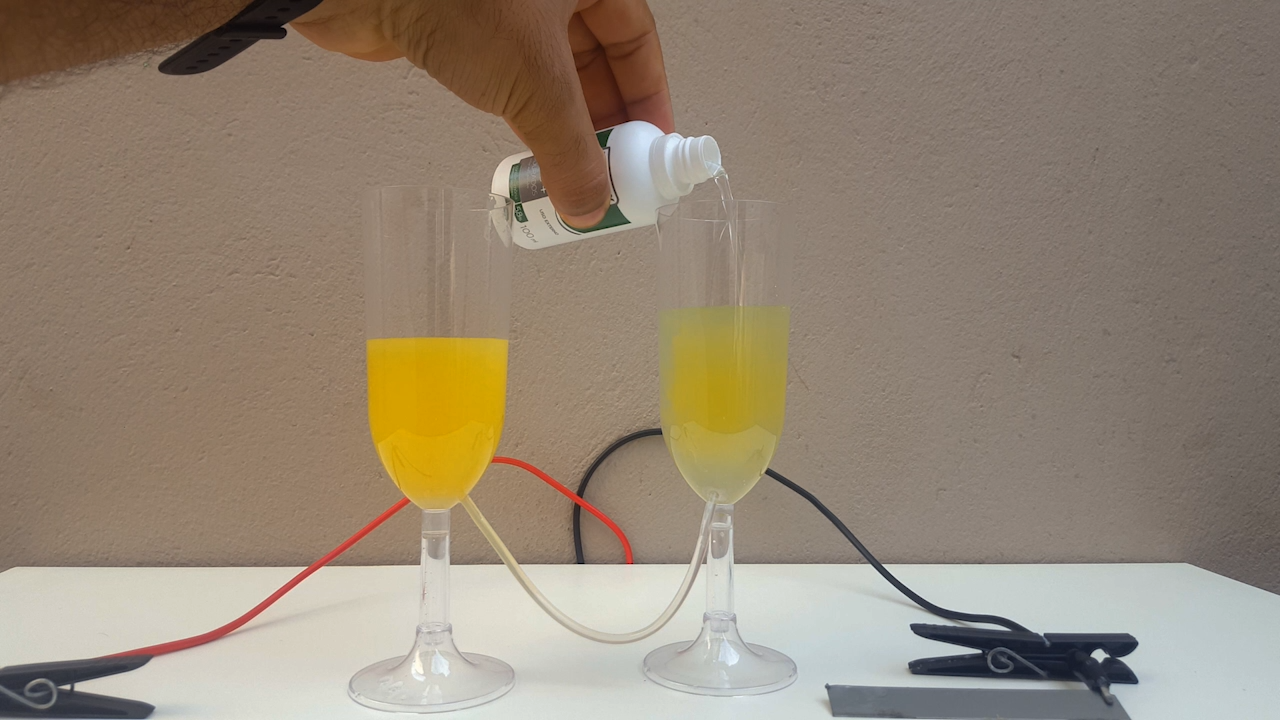
time, CuCl accumulates in the anolyte and, allowing the liquid to flow from one compartment to another, CuOH precipitates. It is a very beautiful
color, ranging from bright yellow to solid orange (photo 02)
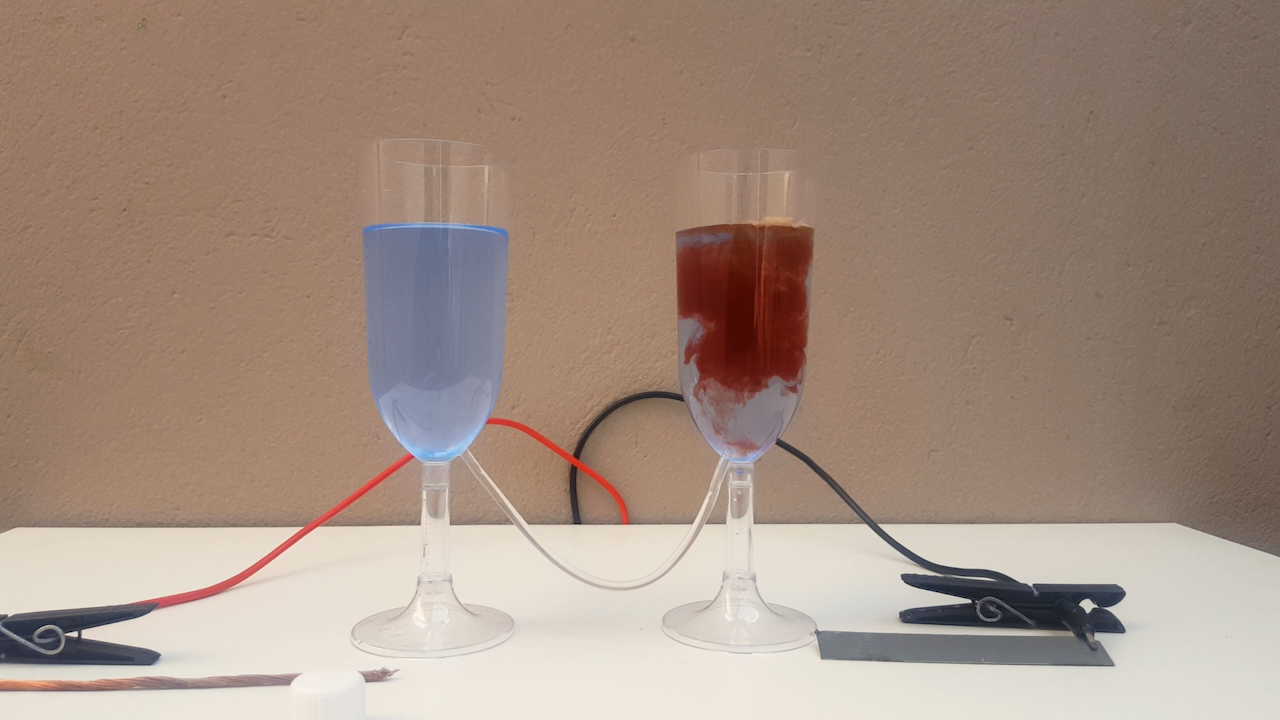
When adding ammonia to the produced CuOH suspension, the liquid changes from a solid, opaque color to the "soft blue" of the complex formed,
presumably diamminecopper, [Cu(NH3)2] + (thanks to DraconicAcid for helping me find out the name of the complex formed by the
interaction of Cu(I) and NH3).
(photos 03 to 06)
Finally, in order not to "waste" the remaining solution, a small fragment of CaC2 is thrown into the flask, which causes copper acetylide to
precipitate.
(photo 07)
But what really caught my attention was the formation of CuCl at the anode. As I wrote earlier in the post "Reagent Production by Electrolysis - a few
ideas" :
[...]"I am very interested in understanding the mechanism by which this occurs. My research in this regard has been unsuccessful. When
electrolysing a concentrated NaCl solution in a cell divided by a salt bridge, using copper anode and a steel cathode operating at 30V, I obtained a
suspension of CuCl in the anode and only traces of CuCl2.Why does it happen? My first theory was that "nascent chlorine", that is, monatomic chlorine,
was combining directly with the copper at the anode. But it seems to me that the "nascent state" theory is already outdated in chemistry and was
abandoned decades ago. In fact, I was so curious about the prevalence of CuCl in this process, since this is the most unstable copper chloride, that I
invested my last free afternoon trying to find the mechanistic explanation of why this happens. Does anybody know?"
The user "Bestbmc" argued the following:
I may have a guess. Assuming chlorine is the limiting reagent, as it either reacts or bubbles off, there is a excess of copper.The standard
enthalpy of formation of copper(1) chloride is -138KJ/Mol, according to NIST, compared to copper(2) chloride, is -205KJ/Mol. Because of this, it is
more favorable for the Cl2 molecule to react and form 2CuCl which a enthaply of formation of -276KJ per mole of Cl2, rather than CuCl2 with a enthalpy
of formation of -205KJ per mol of Cl2. If any CuCl2 is formed at the anode, I would guess it would react with copper metal and be reduced to
2CuCl.
Which struck me as a very reasonable explanation. Any more possibilities?

|
|
|
DraconicAcid
International Hazard
    
Posts: 4356
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
If you have a very concentrated solution of chloride ion, then the reaction CuCl2 + Cu + 2 Cl(-) --> 2 CuCl2(-) becomes favourable, as the chloro
ligands stabilize the Cu(I) oxidation state.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Great experiment and beautiful pictures, which stand out in their simplicity and clean background.
DraconicAcid is right. It is the coordinating property of the chloride ion, which makes the formation of copper(I) favourable. Another experiment,
which very nicely demonstrates this is dissolving some CuSO4 or CuCl2 in conc. HCl. This gives a yellow/brown solution of the CuCl4(2-) complex. When
an excess amount of copper is added to this liquid and the liquid is carefully protected from air, then you first see formation of a very dark brown,
nearly black, mixed oxidation state complex, which contains both copper(I) and copper(II). If there is an excess amount of copper metal, then the
liquid turns colorless, due to formation of the colorless complex CuCl2(-). As soon as you allow air to get in contact with this colorless liquid, you
get the dark brown mixed oxidation state complex again.
Btw. the pale blue color in your experiment is not the color of the Cu(NH3)2(+) complex. That complex is really colorless. The pale blue color is due
to the presence of some Cu(NH3)4(2+). Because of oxygen from the air (or dissolved in the solutions and the ammonia you used), some copper(I) is
oxidized to copper(II), leading to the formation of the blue complex.
See also: https://woelen.homescience.net/science/chem/solutions/cu.htm...
|
|
|
Johnny Cappone
Hazard to Self
 
Posts: 74
Registered: 10-12-2020
Location: Brazil
Member Is Offline
|
|
Thanks for the clarifications!
@Woelen, the curious thing is that I visited this page of your site before running this experiment, while researching the existence of other possible
yellow copper compounds (by the way, apparently there is just one more, the CsCuCl3 complex, whose preparation you recorded in your site). While
browsing there, I read your statement about Cu(NH3)2 being colorless and I was in doubt, since the few references I could find in the literature
described the ammoniacal solution of cuprous chloride used in the synthesis of copper acetylide and/or how a test to indicate the presence of
acetylene as having a deep blue similar to the tetramminecopper complex. Your explanation makes sense, as it is practically impossible to avoid air
during preparation.
Just to satisfy my curiosity, I will reduce some CuCl2 and try to prepare the colorless solution of the diamminecopper complex 
|
|
|
|