Serybva
Harmless

Posts: 13
Registered: 1-5-2018
Member Is Offline
Mood: No Mood
|
|
TLC results interpretation
Hi guys, I'm a, not particularly experienced, amateur chemist and I needed to synthetize ethyl acetate however because I didn't want to spoil my
precious, hardly acquired, 99% ethanol I decided to synthetize Benzyl acetate for fun, training and because it smells pretty good.
The workup was messy because I failed to identify which phase was which, and did 3 tries each time varying a bit the parameters (H2SO4 quantity and
reflux time mostly) thinking maybe my product hydrolysed back to acetic acid and benzyl alcohol...
When I finally understood which phase was the right one I recovered the benzyl acetate phase from the "garbage", distilled it, throwed the first
fraction, kept the second one (the one distilling between 200 and 205°c) which I though was unreacted benzyl alcohol since everytime I tried the
reaction I used a large excess of benzyl alcohol, and kept the third (between 205° and 215°C) thinking this was my benzyl acetate.
I ran a TLC to confirm this and actually my 2nd and 3rd fractions are the same thing, and don't seem to contain any benzyl alcohol, I ran 2 more TLC
with acetic acid and water as reference as well and impurities seem to be none of the 3.
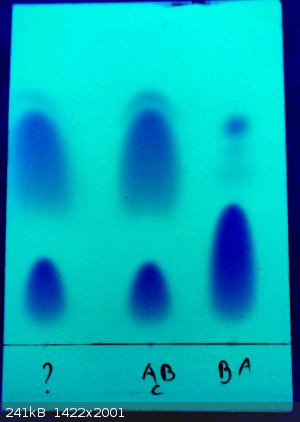
Here's the first one (? is the 2nd fraction, AcB the 3rd and BA is benzyl alcohol):

First question is, how many impurities do I have in my product, one, or two?
If you look closely just above the "highest" spot of the samples there is a small thin croissant shaped spot preceding the big one, is it the same
thing than the one below, or something else?
Second question, is the bottom spot benzyl alcohol or not?
I think I know it's not, but they really look the same and I find odd that I don't seem to have benzyl alcohol in my final product, especially after
only 1 distillation where I kept the distillate coming over between 200° and 215°C
3rd question, after a paper I found online in which authors give Rf values (which i don't remember precisely) for benzyl alcohol and benzyl acetate
using approximately the same eluent solvent I used for their TLC, it seems that my benzyl acetate is the middle spot, but I can't be sure.
Could the spot size, shape and "concentration" be used to determine which is the major compound of the mixture?
4th question, I already knew the benzyl alcohol I used wasn't super pure, though it's said to be 99.9%, I just don't realize how impure it is, what do
you think? Is it still ok or is it like 90% pure?
Thank you all
[Edited on 12-5-2021 by Serybva]
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
The benzyl alcohol may contain a small amount of benzaldehyde. That may be what the smaller, faster moving spot in that lane is. Your spots are
trailing a lot. I recommend redoing the TLC but with smaller, less concentrated spots. They should move and separate more cleanly. Acetic acid and
water won't show up under UV on TLC because they are not UV active. You'll pretty much only be able to visualize aromatics and some other highly
conjugated molecules by UV. Also, if you could resize your picture so it's easier to see and doesn't blow the margins of the thread, that would be
great.
|
|
|
Serybva
Harmless

Posts: 13
Registered: 1-5-2018
Member Is Offline
Mood: No Mood
|
|
Thanks Texium, will try that !
|
|
|
wg48temp9
National Hazard
   
Posts: 786
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Here is a better pic of the TLC plate, the right size and the luminosity has been stretched to increase the contrast.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Oxy
Hazard to Others
  
Posts: 140
Registered: 1-12-2020
Member Is Offline
|
|
Quote: Originally posted by Serybva  | Hi guys, I'm a, not particularly experienced, amateur chemist and I needed to synthetize ethyl acetate however because I didn't want to spoil my
precious, hardly acquired, 99% ethanol I decided to synthetize Benzyl acetate for fun, training and because it smells pretty good.
|
You don't need 99% ethanol for that, you can use 95% if you have access to that. The yield will be lower but still good.
You can test it in a really simple way. If your organic layer/product has low water solubility (or is insoluble) you can take a test tube or beaker,
fill it with water and then put a one drop of the phase you're testing. If you see that it disappeared then you know that it's not your product. If
you see it stays on the top (or bottom) and is not mixing with water then you know it's your ester.
I recommend smaller concentrations for TLC as Texium mentioned. Melting point capillary tubes are good for spotting the samples.
|
|
|
Serybva
Harmless

Posts: 13
Registered: 1-5-2018
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by wg48temp9  | Here is a better pic of the TLC plate, the right size and the luminosity has been stretched to increase the contrast.
|
Thank you this is really nice !
Quote: Originally posted by Oxy  | Quote: Originally posted by Serybva  | Hi guys, I'm a, not particularly experienced, amateur chemist and I needed to synthetize ethyl acetate however because I didn't want to spoil my
precious, hardly acquired, 99% ethanol I decided to synthetize Benzyl acetate for fun, training and because it smells pretty good.
|
You don't need 99% ethanol for that, you can use 95% if you have access to that. The yield will be lower but still good.
|
Yes but I was afraid to get a crappy yield with 95% ethanol, actually the hard part isn't to get 99% ethanol since I just dumped a couple handful of
3A molecular sieves into the bottle, the hard part was the whole fermentation/3 distills process to obtain 95% ethanol, 2 weeks fermentation, 1 day
for the distillation, quite a time consuming route.
In the end I've obtained ethyl acetate with a 46.3% yield, I plan to synthetize acetic anhydride to improve the yield
When I run out of yeasts I think I'll just hydrolyze some ethyl acetate found online with NaOH
Quote: Originally posted by Oxy  |
You can test it in a really simple way. If your organic layer/product has low water solubility (or is insoluble) you can take a test tube or beaker,
fill it with water and then put a one drop of the phase you're testing. If you see that it disappeared then you know that it's not your product. If
you see it stays on the top (or bottom) and is not mixing with water then you know it's your ester.
I recommend smaller concentrations for TLC as Texium mentioned. Melting point capillary tubes are good for spotting the samples.
|
Thanks for the tip !
Toothpicks also work pretty well for that purpose
|
|
|
Oxy
Hazard to Others
  
Posts: 140
Registered: 1-12-2020
Member Is Offline
|
|
| Quote: |
the hard part was the whole fermentation/3 distills process to obtain 95% ethanol, 2 weeks fermentation, 1 day for the distillation, quite a time
consuming route. | Quote: |
Yeah, that's true. I just bought mine as the time and lobour needed to get EtOH by fermentation is really long.
|
|
|
|
|
Serybva
Harmless

Posts: 13
Registered: 1-5-2018
Member Is Offline
Mood: No Mood
|
|
Hi everyone, here it is:

Doesn't make much difference, though I used a concentration about 3 times smaller than in the other one, maybe I'll try another one with even smaller
concentrations like 5 to 10x smaller what do you guys think?
Edit:
Nevermind I did it anyway, with even 3 times smaller sample than this one above, or 9x smaller than the first one:

So it seems that I just have 1 impurity in my benzyl acetate, what could it be?
[Edited on 16-5-2021 by Serybva]
|
|
|
maldi-tof
Harmless

Posts: 45
Registered: 3-4-2019
Member Is Offline
|
|
About benzyl alcohol, as it has been told here, benzaldehyde is the main impurity.
However, benzaldehyde can react with benzyl alcohol to form benzaldehyde dibenzyl acetal. It has a veeeery high boiling point, but...who knows.
If you run a TLC only of your raw materials, maybe you can see if these impurities that you are seeing are being produced, or they are in the some of
your RM.
|
|
|
Serybva
Harmless

Posts: 13
Registered: 1-5-2018
Member Is Offline
Mood: No Mood
|
|
Hey guys, I cheated a bit on this one, a friend of mine who works in a perfume company was kind enough to pass a sample through GC-MS analysis, turns
out I have a mixture of:
- Benzaldehyde (1.08%)
- Benzyl alcohol (36.56%)
- Benzyl acetate (57.63%)
- Dibenzyl ether (3.63%) -> no idea where this one comes from
and 1.1% of unknown other impurities
I now have a better idea of how I'm going to purify those 57.63% of benzyl acetate
|
|
|
Mush
National Hazard
   
Posts: 633
Registered: 27-12-2008
Member Is Offline
Mood: No Mood
|
|
2 Benzyl alcohol (+H2SO4)-> Dibenzyl ether
You kept the benzyl alcohol in excess opposite what needed. Acetic acid needto be in excess to shift the reaction in the direction of benzyl
acetate.
This is the main reason you got all sorts of byproducts.
Purify the benzyl alcohol first , u will get much better results.
[Edited on 25-7-2021 by Mush]
|
|
|
Quieraña
Harmless

Posts: 38
Registered: 24-8-2019
Member Is Offline
|
|
If you have ethyl acetate then you have access to Pure alcohol utilizing sodium hydroxide with ethyl acetate and then distilling the product over as
ethyl hydroxide which is ethyl alcohol or ethanol. That should save you some time to get to that precursor for what you're seeking with respect to
benzyl acetate. I do hope this helps even though it doesn't directly answer your question.
|
|
|