| Pages:
1
2 |
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Aldehyde oxidation
I am going to try the reaction outlined in this paper, but I don't have any acetonitrile to use as a solvent. Is acetonitrile necessary here? The solution is acidic anyway. Can isopropanol
be used instead, keeping the temperature low to prevent side reactions?
Reflux condenser?? I barely know her!
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
No. Hypochlorite will oxidize secondary alcohols to ketones. Acetone might be worth a try.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
I was avoiding acetone because it reacts violently with hypochlorite. I think isopropanol should hold up against hypochlorite at near room
temperature.
Reflux condenser?? I barely know her!
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I suspect otherwise, but I've never tried it. Isopropanol will stand up to chlorite (which is a weaker oxidizing agent) in a Pinnick oxidation of an
aldehyde, but chlorite is harder to get than hypochlorite.
If you're going to try it, stay safe.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Any tips for making concentrated Ca(OCl)2 solutions?
Reflux condenser?? I barely know her!
|
|
|
AvBaeyer
National Hazard
   
Posts: 651
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
There is no obvious reason you could not just use acetic acid as the solvent in place of the acetonitrile. The acetonitrile is probably being used to
ensure solubility of the aldehyde as the reaction proceeds. There are references (not immediately at hand) that add sodium hypochlorite to acetic acid
solutions of the substrate to be oxidized. After all, when the acetic acid is added to the calcium hypochlorite you are generating an aqueous acetic
acid solution of hypochlorous acid. Try looking up oxidation of aldehydes with sodium hypochlorite.
I strongly urge you to not use isopropanol or acetone for this reaction.
AvB
[Edited on 11-5-2021 by AvBaeyer]
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
If you want to make some strong NaOCl solution, try adding pool shock to sodium carbonate. Most of everything precipitates out, leaving ~20% NaOCl. I
think that was in a thread somewhere, about anthranilic acid. Ah, here is the thread: https://www.sciencemadness.org/whisper/viewthread.php?tid=15...
Look for the 4th post. NaOCl solution is probably better than direct pool shock, since that's going to be pretty impure with calcium chloride and
carbonate filler.
[Edited on 11-5-2021 by Triflic Acid]
There wasn't a fire, we just had an uncontrolled rapid oxidation event at the power plant.
|
|
|
Pinnick
Harmless

Posts: 10
Registered: 20-4-2021
Member Is Offline
Mood: aqueous
|
|
Quote: Originally posted by AvBaeyer  | There is no obvious reason you could not just use acetic acid as the solvent in place of the acetonitrile. The acetonitrile is probably being used to
ensure solubility of the aldehyde as the reaction proceeds. There are references (not immediately at hand) that add sodium hypochlorite to acetic acid
solutions of the substrate to be oxidized. After all, when the acetic acid is added to the calcium hypochlorite you are generating an aqueous acetic
acid solution of hypochlorous acid. Try looking up oxidation of aldehydes with sodium hypochlorite.
I strongly urge you to not use isopropanol or acetone for this reaction.
AvB
[Edited on 11-5-2021 by AvBaeyer] |
Hypochlorites are not stable under acidic conditions, throwing Ca(OCl)2 (or any other hypochlorite) into acetic acid will just form calcium acetate
and chlorine gas.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2799
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
^slowly. In a cold solution it will give metastable AcOCl. It's already used in the paper, after all!
But the freezing point of GAA is rather high, and a mixture of AcOH/H2O with no other solvents is generally a poor solvent for organic chemicals. So
that may not be fully applicable.
Dimethyl carbonate is available as a low VOC cleaning solvent. It may be an effective cosolvent for this system, lowering the freezing point and not
being too polar.
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Seems like hypochlorite doesn't form stable solutions with many common solvents, so I think I'll be looking for safer and accessible
over safest and complex. So what about even lower alcohols like ethanol/methanol? I have conflicting reports on the safety of
hypochlorite with lower alcohols, most saying they are reasonably safe at low temperatures.
edit: I was leaning towards using calcium hypochlorite for an easier workup, but might have to go with sodium hypochlorite instead.
[Edited on 5-11-2021 by njl]
Reflux condenser?? I barely know her!
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2799
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Methanol is the least susceptible to oxidation. I don't recommend it at all, but if you had to choose an alcohol, first choice is tBuOH, second is
methanol.
However, as I mentioned, Me2CO3 is probably better and probably available.
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Luckily enough I have some Me2CO3. However, it is mixed with 1,3 dioxolane and I am not in a position where distillation is possible. I would like to
take your advice but right now I will have to try a small scale test with methanol.
edit: For those interested, one problem I ran into during a small test using IPA and aq. NaOCl was phase separation, either due to the polarity of the
hypochlorite solution itself or because of the NaCl product.
[Edited on 5-11-2021 by njl]
Reflux condenser?? I barely know her!
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by Pinnick  | Quote: Originally posted by AvBaeyer  | There is no obvious reason you could not just use acetic acid as the solvent in place of the acetonitrile. The acetonitrile is probably being used to
ensure solubility of the aldehyde as the reaction proceeds. There are references (not immediately at hand) that add sodium hypochlorite to acetic acid
solutions of the substrate to be oxidized. After all, when the acetic acid is added to the calcium hypochlorite you are generating an aqueous acetic
acid solution of hypochlorous acid. Try looking up oxidation of aldehydes with sodium hypochlorite.
I strongly urge you to not use isopropanol or acetone for this reaction.
AvB
[Edited on 11-5-2021 by AvBaeyer] |
Hypochlorites are not stable under acidic conditions, throwing Ca(OCl)2 (or any other hypochlorite) into acetic acid will just form calcium acetate
and chlorine gas. |
Only if there are no other reducing agents for it to react with, which is why acetic acid and hypochlorite is such a common oxidizing agent.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Well, the question is; What Aldehyde do you wish to oxidize?
And, even if you did have Acetonitrile, is this really the best procedure?
I'm not terrified of Acetonitrile, but I am aware that it should be utilized with caution.
Low Boiler. Plenty Flammable. Surprisingly poisonous.
Is Sodium Perborate in Acetic Acid, not suitable for your goals?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Do you have a prep for this?
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Proposed reaction scheme, .1 M scale:
A 250 mL beaker is charged with 14 g Ca(OCl)2. 150 mL room temperature water is added and the beaker is heated to around 90 C. The solution is
thoroughly stirred, then set aside to settle and cool. When Settled and cool to the touch, the solution is decanted and the solids discarded. 15 mL
glacial acetic acid is added dropwise with stirring. The solution is now ready for use.
In a 500 mL beaker, .1 mol aldehyde is dissolved in 100 mL methanol. This solution is stirred and the hypochlorite solution is slowly added, so as to
minimize temperature jumps. After complete addition the reaction mixture is stirred overnight (~12 hours).
Partially thought out workup:
The reaction mixture is filtered. The filtrate is covered and cooled in a freezer. The solution is again filtered. Saturated Na2CO3 solution is added
to precipitate calcium. The solution is filtered, and then brought to neutral pH with HCl. The filtered liquid is then heated to remove methanol. This
should leave only NaCl and the desired acid. The volume is brought down to 100 mL and an equal volume of cold acetone is mixed in. The solution then
filtered.
The first obstacle here is the solubility of the product in water, methanol, and acetone both as a salt and as the free acid. If the product is
insoluble in the methanol/water solvent system then some will be collected after the first and second filtrations. Adding Na2CO3 will surely turn any
dissolved product into an even more soluble salt. Once calcium is removed there will be NaOCl, Na2CO3, and the Na salt of the product in solution. The
product is freed with acidification, and the first 2 side products are decomposed to NaCl. If the product is insoluble in water or acid, it should
precipitate here.
Actual reaction:
16 grams (.11 moles) Ca(OCl)2 from a fresh package and 120 mL tap water were swirled in a 500 mL beaker. 24 grams of glacial acetic acid were added
slowly, with stirring. [note, surprisingly no heat needed for satisfactory dissolution] The solution is decanted into a 250 mL beaker, the 500 mL
beaker is rinsed, and then the solution is returned to the 500 mL beaker.
The aforementioned 250 mL beaker was rinsed, and 22 grams (.11 moles) of aldehyde was added. Then, 150 mL methanol is added and the solution is
stirred to complete dissolution.
The hypochlorite solution is then added in portions to the aldehyde solution with stirring. Starting at 20 C, at no point during the addition did the
temperature ever exceed 35 C. The aldehyde solution quickly turned brown after additions. At a rate of about 20 mL hypochlorite every 2 minutes, the
solution went from an almost crystal clear liquid to more light golden/honey colored, then finally to a more brown, thick honey color. After about 1/2
of the hypochlorite was added, additions began to produce short lived clouds of white precipitate. With each addition they began to linger more. I
didn't want to have hypochlorite floating around not reacting, so I added glacial acetic acid by the milliliter between additions. Eventually there
was too much liquid in the beaker and the reaction mixture was transferred into the 500 mL beaker.
That's where I am now, currently stirring at rt.
Update: Solution was stirred for 4 hours. Immediately after stirring is stopped, the solution looks like a brown cloudy
mess. However this soon separates into a small lower layer and an upper aqueous phase. The lower phase is very much like honey in terms of color. When
a small portion of the reaction mixture is added to concentrated HCl, no bubbling is noted. A lower oily layer forms again, but this time is much more
red/orange. The upper aqueous acid phase is a cloudy off white liquid.
Update 2: About 30 grams of NaHCO3 in 100 mL's water was added with stirring. During addition, the solution first took on a pinkish purple color,
until finally reaching a cloudy pink consistency. A further 30 grams of NaHCO3 in 100 mL's water is added, and this yields a dense precipitate.
Update 3: I stopped the first run too early at just 4 hours. Other problem is that I can't check the pH of the solution because it just bleaches the
paper. I didn't take pictures, but this reaction sequence had some of the coolest color ranges I've seen. New run is going as follows:
A 250 mL beaker was charged with 15 grams Ca(OCl)2, then 100 grams of tap water. 15 grams of glacial acetic acid was added with stirring. The acid
speeds up the hypochlorites dissolution. When the solution looked saturated, the liquid was decanted off of the solids into a 500 mL beaker. The
solids were discarded and the beaker washed. The hypochlorite was then returned from the 500 mL beaker to the 250 mL. The 250 mL beaker and
hypochlorite were covered and put in a freezer.
100 mL alcohol was added to a 500 mL beaker with 22 grams aldehyde. Then 15 grams glacial acetic acid is added, and the solution swirled.
With magnetic stirring, hypochlorite was added in 2 10 mL portions, then the rest was added dropwise at ~3 seconds per drip. No major exotherm
occurred here. After complete addition, about 80 grams of alcohol was added, and the solution stirred for 20 minutes. When the stirring is stopped and
the solution settles a lower layer of honey-colored oil forms. The solution was then heated to 60 C with sufficient stirring to form a roughly
homogenous mixture.
That's where we are.
[Edited on 5-12-2021 by njl]
Reflux condenser?? I barely know her!
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Why would you add the acid to the bleach first, instead of the aldehyde? If you acidify the bleach in the absence of a reducing agent, it will lose
chlorine.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Because that's how they do it in the paper I linked.
Reflux condenser?? I barely know her!
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
The colors in the first reaction ranged from purple, light pink, brown, to gray, honey, and red-orange.
Reflux condenser?? I barely know her!
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Which aldehyde were you oxidizing?
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2799
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
It's a very common system for Baeyer-Villiger oxidation. However, in some cases, particularly with benzaldehydes, this gives C-C bond cleavage and a
formate ester.
Quote: Originally posted by zed  | I'm not terrified of Acetonitrile, but I am aware that it should be utilized with caution.
Low Boiler. Plenty Flammable. Surprisingly poisonous. |
Acetonitrile (LD50: 2g/kg; fp: 2 C) is only a little
worse than methanol (LD50: 5g/kg; fp: 12 C). I see no reason it should be avoided.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Okay- the oxidations I have my students do are almost all substituted benzaldehydes, so I guess I'll stick with Pinnick.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Sodium Perborate in Glacial Acetic Acid? Well it is suggested as a newer form of Peracetic Acid Oxidation. But, maybe not quite that simple.
I've got a few papers somewhere or the other. I'll get it to you.
Sodium Perborate is dirt cheap.
https://www.organic-chemistry.org/chemicals/oxidations/sodiu...
Oh, there have to be better references out there somewhere.
I haven't really been happy with what I have come up with so far.
Converts some cinnamic acid esters to epoxy-esters, which of course can he hydrolysed and decarboxylated, to yield either phenyl acetaldehydes or
benzyl methyl ketones.
[Edited on 13-5-2021 by zed]
[Edited on 13-5-2021 by zed]
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Does the oxidation of aldehydes with hypochlorite require water, or is hypochlorite a good enough nucleophile to add to the carbonyl? IE, top or
bottom scheme?
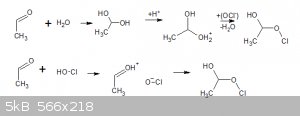
The key to keeping a homogenous solution is using enough alcohol before hypochlorite addition to keep the substrate comfortably in solution as salts
are added. Using 160 grams of alcohol allowed the reaction to run as a homogenous mixture overnight, leaving a transparent brown solution in the
morning. Right now my main issue is the workup, but I'll keep you all updated there.
Update: For those of you following along at home, my next plan for workup is to remove as much alcohol as possible from the reaction mixture and
acidify with HCl, then once it's all settled extract with ether. I want to try a smaller scale reaction, maybe 10 g substrate to 9 grams hypochlorite.
[Edited on 5-18-2021 by njl]
Reflux condenser?? I barely know her!
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Ok no answers there, but I would like to report some success using basic aqueous NaOCl and no additional solvent. The reaction ran smoothly but took
quite a long time, so this seems like a perfect opportunity to test catalysts.
The procedure was (briefly) as follows:
1 eq. aldehyde, 1.75 eq. aq. NaOCl, 2.5 eq. NaOH are added to a beaker with stirring. The temperature was maintained at rt for 12 hours, then held at
~80 C for another 12. Finally, the solution is washed with ether to remove byproducts (the product exists at this pont as the sodium salt, insoluble
in ether). The solution was then acidified with HCl, filtered, and washed with cold water.
I think the key to higher yields will be addition of the aldehyde over time to the oxidizing solution. If it's all added at the beginning a
brown/ornage plastic mass results (melts between 60 and 80 C, insoluble in acid and base, soluble in ether) that slowly shrinks in size as the
reaction progresses.
More coming once I have all the info together.
[Edited on 6-4-2021 by njl]
Reflux condenser?? I barely know her!
|
|
|
| Pages:
1
2 |