Biotech_Yossorab
Harmless

Posts: 13
Registered: 5-12-2018
Member Is Offline
|
|
Dibal-H in THF to alcohol mechanism
Hello,
I'd like to have some help with my mechanism. I've been scratching my head trying to understand how the Dibal-H can reduce up to the alcohol and where
do that one hydrogen comes from.
I've found a website where THF is actually helping the reaction by creating a weakening in the bond
http://www.chemgapedia.de/vsengine/vlu/vsc/en/ch/2/vlu/oxida...
I'm fairly certain about the first steps of my mechanism but I don't know where the hydrogen comes from to make the alcohol...
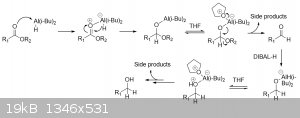
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
The first step is the formation of a lewis adduct between the ester carbonyl and Dibal-H (the carbonyl lone pair being the lewis base and the aluminum
center being the lewis acid). The hydride on the aluminum center then attacks the carbonyl carbon which is conveniently situated close to the hydride,
forming a neutral aluminum alkoxide. THF forms another complex with the aluminum center and facilitates its removal from the carbonyl oxygen. What is
left is a hemiacetal, which rearranges with loss of R2OH to give an aldehyde. The same mechanism is then repeated on the newly formed aldehyde
carbonyl, except this time followed by protonation of the alkoxide to give an alcohol.
The sequence of hydride attacking carbonyl carbon -> formation of alkoxide with hydride counterion -> hydrolysis of alkoxide is a
common motif among carbonyl reductions with hydride or hydride equivalents. The details of the mechanisms are different for every reaction but the
overall idea is common to several.
Reflux condenser?? I barely know her!
|
|
|
Biotech_Yossorab
Harmless

Posts: 13
Registered: 5-12-2018
Member Is Offline
|
|
Quote: Originally posted by njl  | The first step is the formation of a lewis adduct between the ester carbonyl and Dibal-H (the carbonyl lone pair being the lewis base and the aluminum
center being the lewis acid). The hydride on the aluminum center then attacks the carbonyl carbon which is conveniently situated close to the hydride,
forming a neutral aluminum alkoxide. THF forms another complex with the aluminum center and facilitates its removal from the carbonyl oxygen. What is
left is a hemiacetal, which rearranges with loss of R2OH to give an aldehyde. The same mechanism is then repeated on the newly formed aldehyde
carbonyl, except this time followed by protonation of the alkoxide to give an alcohol.
The sequence of hydride attacking carbonyl carbon -> formation of alkoxide with hydride counterion -> hydrolysis of alkoxide is a
common motif among carbonyl reductions with hydride or hydride equivalents. The details of the mechanisms are different for every reaction but the
overall idea is common to several. |
Thank you for your response. So that means this reaction always occurs in presence of moisture/water? It's kind of strange considering that the paper
this reaction is from never mentions water anywhere.
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
No, the reaction produces an alkoxide, and the alcohol’s proton comes from aqueous workup once the reaction is complete. Similarly to Grignard
reactions and other reductions that use strongly basic reagents.
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
No, sorry for not being clear. The majority of the reaction takes place solely in non-protic solvents. This is common among nearly
all of the reactions of this type, since water or any proton sources can interfere with the reaction. For example, if water is present in the reaction
mixture it will generally destroy the organometallic reagent being used including DIBAL-H. The premise of these reactions is that a nucleophilic
species (hydrogen in the case of a reduction or and aklyl group for Grignard/Barbier/etc.) is being carried to the electrophile (carbonyl carbon)
where the two combine to form an alkoxide (the cation of which depends on the reagents used). Alkoxide is then hydrolyzed to the product. If water is
present it will be attacked since water (and the resulting hydroxide from deprotonation) are better ligands than hydride. The key is that you can run
a reaction and then hydrolyze the alkoxide all at once, there's no need for any protic material until you are finished with the reaction. That's why
Grignard reactions and lithium aluminum hydride reductions are quenched with water or acid upon completion. Because the actual products of the
Grignard and the reduction are not the desired alcohols, they are the corresponding alkoxides.
Your case (reduction of an ester to primary alcohol with DIBAL-H) is very slightly complicated by the fact that there are actually 2 reduction steps
going on (ester->aldehyde->alcohol). The second step cannot begin until the product of the first is turned into its carbonyl form. Taken at face
value this would imply that the reduction must be carried out in more steps so that the first reduction product can be isolated and hydrolyzed.
However, the THF solvent allows the product of the first step to eliminate an alcohol which negates the need for protonation. Other
ether solvents (technically lewis bases in general) also allow elimination. This allows you to carry out the complete reduction of an ester to a
primary alcohol in one pot without isolation of any intermediates. As previously mentioned, the last step in your reaction before workup will be to
quench the reaction mixture with a proton source to free your final product.
Notes: I only know what I have taught myself so take this with a grain of salt (though I am fairly confident in this explanation). Specifically
however I'm not sure my explanation of why water destroys the organometallic reagent is correct. Anyway I'm done rambling, I hope this made sense.
Reflux condenser?? I barely know her!
|
|
|
Biotech_Yossorab
Harmless

Posts: 13
Registered: 5-12-2018
Member Is Offline
|
|
Thanks, both of you. Yeah, I think my question wasn't really clear but your explanation is great.
I knew the whole reaction was taking place in dry THF, I just didn't understand where that final proton came from, since neither acid nor water was
mentionned in any work-up. I thought the hydrogen came from the DIBAL-H itself and didn't make any sense, ence my difficulty to understand the
reaction.
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Right, the final proton on the alcohol does not come from the DIBAL-H. If no quenching/protonation is in the procedure, they may have glossed over it
and assumed that those following their method would assume it is needed, or it could be included in their work up.
Reflux condenser?? I barely know her!
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
Draw your 'side product' in the first step you will note that it is Diisobutylaluminium methoxide. No protons needed. No THF invoked in the mechanism.
In short the tetrahedral intermediate you've drawn (the acetal) is only stable at lower temperatures and can decompose (or collapse if you will) into
said DIBAL-OMe and the aldehyde.
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
You should be more careful before discounting solvent effects in a reaction like this. Ethereal solvents and THF in specific are known to catalyze
such reactions. However unstable the intermediate is, methoxide is hard to eliminate without some persuasion.
Reflux condenser?? I barely know her!
|
|
|
Texium
Administrator
       
Posts: 4618
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
THF is certainly not required though. I’ve run DIBAl-H reductions from methyl ester to alcohol in DCM many times with no issues and high yields.
|
|
|