Biotech_Yossorab
Harmless

Posts: 13
Registered: 5-12-2018
Member Is Offline
|
|
Which of these aldehydes are more reactive
I'm working on this reaction (see below) and I've been wondering if the negatively charged oxygen could react with the second aldehyde instead [18]
after adding LHMDS. If not, why wouldn't it?
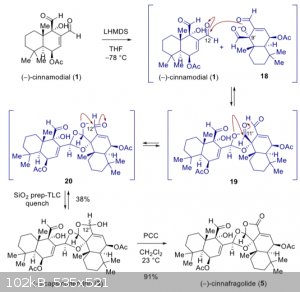
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Very complicated. You don't know if this reaction works?
Where is this from?
|
|
|
Pinnick
Harmless

Posts: 10
Registered: 20-4-2021
Member Is Offline
Mood: aqueous
|
|
I think there are a couple of things going on here.
An aldehyde is attacked in a certain angle (https://en.wikipedia.org/wiki/B%C3%BCrgi%E2%80%93Dunitz_angl... ).
This means that the aldehyde wich is less sterically hindered will be attacked preferentially wich seems to be the one depicted here (C12).
It is likely that both aldehydes are attacked, but the reaction conditions are optimized for only one of them (thermodynamic/kinetic control for C12).
The low temperature might indicate this.
Another factor could be the next three intramolecular attacks [19+20]. These are reversible and could occur by attacking the second aldehyde aswell.
They might just be less stable so the transition state is more easily converted back to the educts. It is also possible that the attack on the second
aldehyde will not result in these intramolecular reactions and therefore not stabilize the compound so it reacts back.
The truth is probably hidden in all assumptions and you have made a good point: there is defenetly some reaction involving the second aldehyde. This
is a complicated molecule and a lot can happen, indicated by the yield.
|
|
|