| Pages:
1
..
3
4
5
6
7
8 |
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Lithium violurate

|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Gallium violurate. It has more light colour than indium violurate.

|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Quote: Originally posted by Bezaleel  |
Indium
It seems that indium is chemically too basic to easily form a salt with violuric acid. My estimate is that this will count all the more for Re, Mo and
W. I decided that I will not make the uranyl salt, because of the precautions necessary to safely work with it. I consider cerium, which is
interesting because it is a coloured RE and also has a stable +IV oxidation state, and aluminium. |
I did it easily, dissolving the carbonate in acid
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 202
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
Since there have now been so many metal salts made, i put together a periodic table . There is still a lot of them to be made 
group 1 is complete!
for group 2 only beryllium is missing.
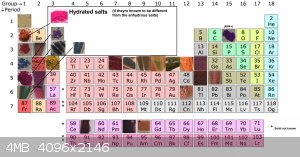
[Edited on 27-3-2021 by RustyShackleford]
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Perfect. I have beryllium i will make it
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Mercury(I) violurate. I used mercury(I) nitrate. I used excess nitrate. The required amount was dissolved in the acid and the excess crystals stayed
undissolved. Then a pink suspension was formed, I poured it into another flask and filtered it.

|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Divide the hydrate plate and for example bivalent mercury is inserted there. Or think of something else. I really like periodic table idea.
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Quote: Originally posted by vano  | Quote: Originally posted by Bezaleel  |
Indium
It seems that indium is chemically too basic to easily form a salt with violuric acid. My estimate is that this will count all the more for Re, Mo and
W. I decided that I will not make the uranyl salt, because of the precautions necessary to safely work with it. I consider cerium, which is
interesting because it is a coloured RE and also has a stable +IV oxidation state, and aluminium. |
I did it easily, dissolving the carbonate in acid
|
I find it odd to say the least that the carbonate dissolves easily, whereas the hydroxide takes quite some time to fully dissolve. In particular, my
hydroxide was not heated and was always in a liquid, so it never dried. It should be easily reacting.
Quote: Originally posted by RustyShackleford  | | Shame that the indium didnt form a solid, the solution looks real nice though. Also im quite suprised the Sm and Pr are so similar in color, from all
the other salts i woulve expected a bigger difference. They both look quite nice though, thank you for taking the time to produce the salts and post
pictures! |
It did form a solid. Two, actually. After filtering off the excess of indium hydroxide, the solution first gave a crop of bright red coloured
crystals, while the solution turned from purple-blue to a deep dark blue.
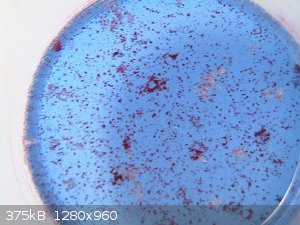
At one point I noticed that no more crystals appeared, although more of the liquid had evaporated. I then poured off the blue liquid and continued to
evaporate the dark blue liquid over NaOH (as before). When almost no liquid had remained, dark blue crystals formed. When standing longer in the
desiccator, they turned off-white, like the colour of violuric acid itself. On standing outside the desiccator for a few days, the dark blue colour
came back. The photo below shows the red and blue compounds I obtained.

I was unsure whether the blue or red compound maybe was ammonium violurate, since I used ammonia to make the indium hydroxide. To double check, I took
the indium hydroxide which had not dissolved in the experiment. It was rinsed on a filter, until almost all of the colour from the dark blue liquid
had washed out. A spatula tip of violuric acid was added to the washed hydroxide and water was added to obtain a purple solution. After stirring on a
70C hotplate for 1 hour, the colour had darkened to a more intense purple. The mixture was poured into a pointed flask to let the residue settle.

The purple liquid was evaporated in a desiccator over NaOH, as usual. To my surprise, this time also red crystals and a blue solution were obtained,
just as in the first experiment.
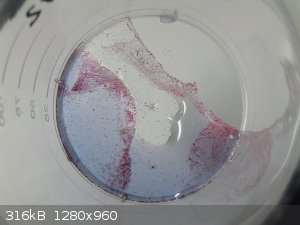
I don't believe that either the red or the blue compound is an ammonium compound, but I haven't done any testing.
Quote: Originally posted by vano  | Thanks. This is indium violurate. Brown- dark red colour. I used indium carbonate. Carbonates are better.
[Edited on 27-3-2021 by vano] |
Could it be that you have a mix of the red and blue solids I got? Is your sample anhydrous?
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Quote: Originally posted by RustyShackleford  | Since there have now been so many metal salts made, i put together a periodic table . There is still a lot of them to be made 
group 1 is complete!
for group 2 only beryllium is missing.
[Edited on 27-3-2021 by RustyShackleford] |
Nice work! 
Maybe it's a good idea to write the element symbols into the pictures.
The europium solution is not representative. It was made from EuCl3, which inhibits formation of larger amounts of Eu-violurate. I regained some
violuric acid from the failed Pr synth. If you wish, I can make the Eu salt from Eu2(CO3)3, or I can try Ce or Er, whichever you prefer.
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
I had indium nitrate solution and i made carbonate from it. Also some metals for example indium produce hydroxyindates and this is why I prefer
carbobate. I also avoided the presence of other cations in the solution as much as possible
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Beryllium violurate.
1)Solution was green
2) hydrated salt was yellow
3) anhydrous is brown
4) When I finished synthesis, I decided to wash the flask, but when I poured water it turned purple. It's pretty weird.
   
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Silver violurate. Solution and anhydrous. I think red particles are hydrates.
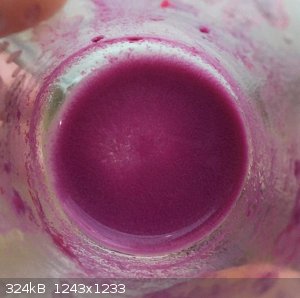 
|
|
|
Texium
Administrator
       
Posts: 4678
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: Preparing to defend myself (academically)
|
|
Quote: Originally posted by vano  | Beryllium violurate.
1)Solution was green
2) hydrated salt was yellow
3) anhydrous is brown
4) When I finished synthesis, I decided to wash the flask, but when I poured water it turned purple. It's pretty weird. |
Beryllium is a strange element. It doesn’t follow the same trend as other alkaline earth metals. Its compounds are covalent rather
than ionic, and readily undergo hydrolysis. Most, if not all, of the pictures that you posted are probably not “beryllium violurate” in the sense
that you’re describing.
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Yes you are right. I think 4 is hydrolyzed compound.
|
|
|
Bedlasky
International Hazard
    
Posts: 1251
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Quote: Originally posted by vano  | Beryllium violurate.
1)Solution was green
2) hydrated salt was yellow
3) anhydrous is brown
4) When I finished synthesis, I decided to wash the flask, but when I poured water it turned purple. It's pretty weird.
|
I think that green and yellow colours are due to some complex formation. Hydrated salt is purple according to behavior in point 4.
How can you be so sure that silver salt is anhydrous? Red colour can be caused by different particle size.
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
The color changed and faded. We have talked about the size and color of the particles before, in this case the red particles were on the walls of the
flask, while the dark bottom, i.e. the red, lost less water.
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Quote: Originally posted by Bedlasky  |
I think that green and yellow colours are due to some complex formation. Hydrated salt is purple according to behavior in point 4.
|
I think its hydrolyzed. I may try to make hydrate in the future as well. This time bivalent mercury and cadmium are in the queue.
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Mercury(II) violurate. I used mercury oxide.

|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Just dilute solution of the acid
There were acid particles on the walls of the package. I added water and got this color, then poured it into a vial. The color is well visible on a
white background. I think the sharp change in color during hydrolysis is also caused by this.
 
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 202
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
Strange, I just tested dissolving some of the very same batch i sent you and its an almost completely clear solution, slight hint of pink (likely some
residual sodium contamination). Perhaps you used a metal spoon or a contaminated plastic one at some point, or maybe the baggie i sent had some
contaminant dust on the inside.

Also here is the updated perodic table of violurate salt.
Thank you Vano for your tremendous contribution!
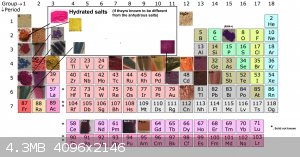
[Edited on 1-4-2021 by RustyShackleford]
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Thank you! It was a good experience. I have never used metal spoon. I always pour the powder directly from the package onto a sheet of paper. If this
was caused by the plastic, since it was inside for a long time, then it is interesting. I rule out the presence of sodium ions in the solution as I
have not used any sodium compounds.
Did you spill silver nitrate on your hand?
[Edited on 1-4-2021 by vano]
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 202
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
yes haha, ive been purifying some teeth amalgam powder recently, i think i touched the inside of the beaker while washing it.
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
 . Once almost half of my hands became black, in the past I was very fond of
separating pure silver from alloys. You know there are ways, but from personal experience it takes about 4 hours to put your hand in the water to
remove the black layer from the skin. . Once almost half of my hands became black, in the past I was very fond of
separating pure silver from alloys. You know there are ways, but from personal experience it takes about 4 hours to put your hand in the water to
remove the black layer from the skin.
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Quote: Originally posted by RustyShackleford  | | Strange, I just tested dissolving some of the very same batch i sent you and its an almost completely clear solution, slight hint of pink (likely some
residual sodium contamination). Perhaps you used a metal spoon or a contaminated plastic one at some point, or maybe the baggie i sent had some
contaminant dust on the inside. |
I consistently get the same results. I use a nickel spatula, but I can
hardly believe that nickel oxide would indeed react with the violuric acid.
Quote: Originally posted by RustyShackleford  | Also here is the updated perodic table of violurate salt.
Thank you Vano for your tremendous contribution!
[Edited on 1-4-2021 by RustyShackleford] |
Great - thanks Rusty!
In the meantime I prepared aluminium violurate, which is another medium red compound, and reddish pink in solution (photos to follow).
I also retested the indium compounds I obtained and it gave me the same results as before. First a crop of red crystals is deposited and after this
has stopped and the solution is left to crystallise in a separate beaker, a crop of blue crystals is formed. On re-dissolving these red and blue
compounds, the same red and blue compounds are formed, so they do not exists in equilibrium with each other. Together with the red compound, some
violuric acid also seems to crystallise out. My guess is that I obtained basic indium compounds and that vano has obtained the normal, non-basic
indium violurate.
 
|
|
|
samarium14
National Hazard
   
Posts: 661
Registered: 22-3-2019
Location: Georgia
Member Is Offline
|
|
Nice work Bezaleel
|
|
|
| Pages:
1
..
3
4
5
6
7
8 |