nezza
Hazard to Others
  
Posts: 324
Registered: 17-4-2011
Location: UK
Member Is Offline
Mood: phosphorescent
|
|
Sulphuric acid
Just a quick question about sulphuric acid. Will 90% sulphuric acid substitute for conc (98%) in its reactions ?.
If you're not part of the solution, you're part of the precipitate.
|
|
|
outer_limits
Hazard to Others
  
Posts: 139
Registered: 3-3-2020
Member Is Offline
Mood: hybridized
|
|
Not for all. In some it's used also as dehydrating agent so the efficiency may be much lower. It's oxidative properties can be also decreased in 90%
concentration.
[Edited on 10-1-2021 by outer_limits]
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
agree with outer_limits, it depends on the reaction.
If you have 90% however put a beaker on a hotplate and heat it to boiling .. that will drive off water preferentially.
Do it outside with good ventiation and use a watchglass to prevent too much splashing.
Cover anything you want to keep with fiberglass cloth.
Fiberglass cloth also works instead of a watch glass.
Use precautions like sand mixed with sodium carbonate around the apparatus.
Use extreme caution and you will have acid that is useful for most dehydrations.
Boiling in open air will also destroy organics but will concentrate metal ions a little.
|
|
|
Deathunter88
National Hazard
   
Posts: 519
Registered: 20-2-2015
Location: Beijing, China
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by macckone  | agree with outer_limits, it depends on the reaction.
If you have 90% however put a beaker on a hotplate and heat it to boiling .. that will drive off water preferentially.
Do it outside with good ventiation and use a watchglass to prevent too much splashing.
Cover anything you want to keep with fiberglass cloth.
Fiberglass cloth also works instead of a watch glass.
Use precautions like sand mixed with sodium carbonate around the apparatus.
Use extreme caution and you will have acid that is useful for most dehydrations.
Boiling in open air will also destroy organics but will concentrate metal ions a little. |
This wont work sadly. Once you get above about 80-85% concentration, you lose almost as much acid as you do water. The only true way to get from 90%
to 98% is distillation.
|
|
|
artemov
Hazard to Others
  
Posts: 181
Registered: 22-8-2018
Member Is Offline
|
|
I boiled battery acid to about 80-82% in an open rbf. By about 75% I have to hold my breath to get near the apparatus, an accidental breath resulted
in a most choking cough and painful throat and lungs.
As you can see from the graph below, from about 70-75% onwards, the concentration of the acid in the vapor starts to become non-negligible.
Will be very prepared next when I try to reach 90-95% in a sealed setup with a tube leading out of the window.
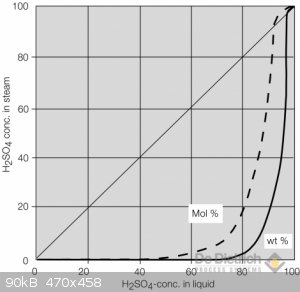
[Edited on 11-1-2021 by artemov]
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
You will need an efficient column to concentrate 90% sulfuric acid any further.
|
|
|
Sulaiman
International Hazard
    
Posts: 3695
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Quote: Originally posted by artemov  | | ... Will be very prepared next when I try to reach 90-95% in a sealed setup with a tube leading out of the window... |
Nooo!
Condense and save all of the distillate that comes over before the fraction that you want to collect.
This is distilled dilute sulphuric acid.
The most concentrated acid is the stuff left in the boiling pot.
I collected distilled dilute acid,
distilled concentrated acid (I guess 95%, not yet titrated),
a small quantity of what seems near azeotropic (again, not titrated)
and some cloudy but very near azeotropic acid left in the pot.
If distilling at atmospheric pressure be very prepared for violent bumping,
Using glass beads my first batch ran very smoothly with no bumping.
Second batch, same glass beads - twice I had violent bumps.
Boiling hot concentrated sulphuric acid ejaculations are very scary and could easily destroy skin, eyes etc.
BE VERY CAREFUL AND WELL PREPARED.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Try pumping air into the boiling acid with a bleeder capillary tube attached to a mini aquarium air pump. Capillaries are very easy to make from any
piece of glass tubing and a torch, perhaps even common flame is enough.
|
|
|
artemov
Hazard to Others
  
Posts: 181
Registered: 22-8-2018
Member Is Offline
|
|
Quote: Originally posted by Sulaiman  |
Nooo!
Condense and save all of the distillate that comes over before the fraction that you want to collect.
This is distilled dilute sulphuric acid.
The most concentrated acid is the stuff left in the boiling pot.
If distilling at atmospheric pressure be very prepared for violent bumping,
Using glass beads my first batch ran very smoothly with no bumping.
Second batch, same glass beads - twice I had violent bumps.
Boiling hot concentrated sulphuric acid ejaculations are very scary and could easily destroy skin, eyes etc.
BE VERY CAREFUL AND WELL PREPARED. |
I will be doing something like this, using a Vigreux as an air condenser. Should I add a liebig (no coolant running) after the Vigreux since my
Vigreux is quite short?
The tube will be connected to the vacuum adapter outlet to lead the excess fumes outside, so I'll be collecting the distilled diluted acid as well. My
battery acid are quite pure, what remains in the distillation flask will be my concentrated acid.
I have small ptfe pieces as boiling chips and will be very very careful, with goggles and respirator 

[Edited on 11-1-2021 by artemov]
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
It will work.
And note the precautions I suggested, outside AND well ventilated with a watch glass or fiberglass cloth.
You need high heat.
Most hot plates are insufficient, with many not even being 200W.
It is messy and dangerous but it will work.
You will lose appreciable acid but the end result will be enough for most dehydrations and will be close to 98% if not exact.
You can always run it longer.
If it could not be done, then distillation would not work either.
And anyone suggesting a fractionating column with sulfuric acid has never attempted to distill sulphuric acid with a fractionating column.
Also you cannot use ptfe when boiling sulfuric acid. It melts. Use glass chips. And you will still get bumping.
Also do not use plastic keck clips, they will also melt.
|
|
|
Sulaiman
International Hazard
    
Posts: 3695
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Quote: Originally posted by artemov  | | I will be doing something like this, using a Vigreux as an air condenser. Should I add a liebig (no coolant running) after the Vigreux since my
Vigreux is quite short? |
I used a plain fractionating column as an air condenser followed by a Leibig condenser with an aquarium air pump 'blowing' air through the jacket.
Even when the sulphuric acid vapours condense, the liquid is at about 300oC,
the air-blown Leibig will help to cool the acid for collection.
I expect that there is a real risk of glassware failure due to thermal gradient stresses,
a Vigreux column 'should' be ok, but any piece with flaws is likely to fail.
Although I've seen videos of empty Leibig condensers being used as air condensers I think that this could easily cause failures.
(inner tube much higher temperature than outer tube so expanded more, lengthways)
PS assemble glassware clean and dry,
condensed vapours will (almost) seal the joints.
Oil or grease will be converted to a tarry glue.
I think that it is a good idea to clean and dry anti-bumping devices
- to re-introduce bubble-forming spots (nucleation points?)
before re-use. I've not confirmed this.
Collecting a little of the hot conc. acid in a disposable pipette,
then applying the acid to various substances,
will instantly persuade anyone of the dangers.
And its fun.
[Edited on 11-1-2021 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
artemov
Hazard to Others
  
Posts: 181
Registered: 22-8-2018
Member Is Offline
|
|
Quote: Originally posted by macckone  | It will work.
And note the precautions I suggested, outside AND well ventilated with a watch glass or fiberglass cloth.
You need high heat.
Most hot plates are insufficient, with many not even being 200W.
Also you cannot use ptfe when boiling sulfuric acid. It melts. Use glass chips. And you will still get bumping.
Also do not use plastic keck clips, they will also melt.
|
I have no "outside", so I will have to do it "inside" lol ... luckily I have windows though ... I also have a heating mantle ...
OMG I totally forgot my diy ptfe boiling chips already sort of stuck together after distilling to 80+% the other time. Thanks for reminding!
Quote: Originally posted by Sulaiman  |
I used a plain fractionating column as an air condenser followed by a Leibig condenser with an aquarium air pump 'blowing' air through the jacket.
Even when the sulphuric acid vapours condense, the liquid is at about 300oC,
the air-blown Leibig will help to cool the acid for collection.
I expect that there is a real risk of glassware failure due to thermal gradient stresses,
a Vigreux column 'should' be ok, but any piece with flaws is likely to fail.
Although I've seen videos of empty Leibig condensers being used as air condensers I think that this could easily cause failures.
(inner tube much higher temperature than outer tube so expanded more, lengthways)
|
Thanks for all the tips and hints ... I dun have an air pump unfortunately, maybe I will start the heating very very slowly, the entire liebig might
have a chance to equilibrate it's temp better. All I need to do is "evaporate" as much water off the acid as possible, however much time it takes, so
I dun really need to boil it vigorously. Either that or I risk running room temp water through the Liebig 
|
|
|
Sulaiman
International Hazard
    
Posts: 3695
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Anyone here actually done a fractional distillation of sulphuric acid ?
I'm now considering it - because I can.
Any comments,
and, is it worth the effort vs. SO3 dissolved in conc. sulphuric acid?
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
teodor
National Hazard
   
Posts: 876
Registered: 28-6-2019
Location: Heerenveen
Member Is Offline
|
|
A good water vacuum aspirator is not expensive and can give 20 mmHg vacuum easily. Could it make the process of fractional distillation of sulphuric
acid achievable, also reducing risk of ejecting SO3 vapours in case of an accident?
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
I would use a double suck-back trap if I was going to do this.
Water and hot sulfuric acid are not going to mix quietly.
A water aspirator will work but it won't reduce the boiling point much.
Sulfuric acid is stubborn stuff to distill.
It may however make it easier to concentrate it before the sulfuric acid reaches full concentration.
|
|
|
artemov
Hazard to Others
  
Posts: 181
Registered: 22-8-2018
Member Is Offline
|
|
Ok so I did my distillation today, with small pieces of broken glass as boiling chips, even so the bumping got really scary. It would be all quiet and
barely boiling, and bam! a sudden burst of opaque white smoke filled the entire apparatus near instantaneously (but I think no hot acid got splashed
over, can't really see through the smoke). I was really worried the glassware/Vigreux would crack).
After 3 or 4 such bumps I got scared and turned off the power 
In the end I got about 90% concentration (density ~1.81, hope it's good enough for esterification, and maybe some drying ...
The good thing is that despite all the smoke, nothing acrid leaked out, so this sort of setup is probably doable for concentrating battery acid to
90-95% ...

[Edited on 18-2-2021 by artemov]
|
|
|
Johnny Cappone
Hazard to Self
 
Posts: 74
Registered: 10-12-2020
Location: Brazil
Member Is Offline
|
|
Quote: Originally posted by macckone  | It will work.
And note the precautions I suggested, outside AND well ventilated with a watch glass or fiberglass cloth.
You need high heat.
Most hot plates are insufficient, with many not even being 200W.
It is messy and dangerous but it will work.
You will lose appreciable acid but the end result will be enough for most dehydrations and will be close to 98% if not exact.
You can always run it longer.
If it could not be done, then distillation would not work either.
|
Yes, it is curious how so many people continue to insist that azeotropic acid cannot be reached by simply boiling it in an open bottle, but only by
distillation.
In fact, if the first form is not effective, it is irrational to imagine that the second would be. It is possible to achieve up to 90% concentration
with "only" 15% acid present in the vapor, from which point the loss of H2SO4 in the vapor increases gradually (and rapidly) until the constant
boiling point is reached.
Certainly some acid is lost, but not so much as to make the process economically unfeasible. I would say that distillation is better and, under the
right conditions, safer; in addition to allowing to recover the most diluted fractions, which can be concentrated again. But it is not the only way to
obtain 98% H2SO4. There is no need for a distillation apparatus for this.
Attachment: Sulfuric Acid-Water - Concentration in vapor-liquid.pdf (328kB)
This file has been downloaded 309 times
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by artemov  | | Ok so I did my distillation today, with small pieces of broken glass as boiling chips, even so the bumping got really scary. |
Glass chips don't make great boiling chips since they are smooth and neither porous nor particularly rough. Rough boiling stones
work much better.
|
|
|
artemov
Hazard to Others
  
Posts: 181
Registered: 22-8-2018
Member Is Offline
|
|
Quote: Originally posted by Texium (zts16)  | Quote: Originally posted by artemov  | | Ok so I did my distillation today, with small pieces of broken glass as boiling chips, even so the bumping got really scary. |
Glass chips don't make great boiling chips since they are smooth and neither porous nor particularly rough. Rough boiling stones
work much better. |
Mine is frosted glass bottle, I thought it would be "rougher"
I wanted to use stones but I was worried the unknown composition might react with hot concentrated sulfuric acid. Pumice has been suggested, but
surely it is too light?
|
|
|
Edward Science
Harmless

Posts: 1
Registered: 26-11-2018
Member Is Offline
|
|
Ceramic, pumice and clay chips all work, although the latter two will be destroyed in hot concentrated sulfuric acid after 5 hours or so, but if you
are doing a distillation it shouldn't be a problem since the leftovers of the boiling chips won't come over. If you are using normal rocks or stones
you should test them with a little acid to make sure they aren't carbonate rocks or it would react. Sand also works to prevent bumping.
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Use ebulliator and air pump. Any glass tube can be drawn into one with hand torch.
Any chips so far I've tested did not work, activated carbon was the best but it reacts when conc gets higher.
|
|
|