metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
Why do Mg salts not color the flame ?
Weird question.
Salts of Ca, Sr, Ba color the flame resp. orange, red, yellowish green. Same applies to the alkali metals.
https://upload.wikimedia.org/wikipedia/commons/4/45/Magnesiu...
And the spectrum of Mg has a clear green line.
So why do Mg salts held in a flame not show a greenish color ? And Mg does burn with bright white rather than green ?
[Edited on 2021-1-22 by metalresearcher]
[Edited on 2021-1-22 by metalresearcher]
|
|
|
Junk_Enginerd
Hazard to Others
  
Posts: 251
Registered: 26-5-2019
Location: Sweden
Member Is Offline
|
|
I'm no expert on this but my reasoning is: Yes, there's a clear green line. Also lots of blue ones and also red ones. Red+green+blue=white.
Our sun is also mainly green, but looks pretty white.
The white when magnesium burns is probably more related to the extreme temperature at which it burns. Copper metal also burns white. It's so hot and
bright that we can't really see any color.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4357
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
When magnesium burns, it's mostly blackbody radiation (although I've long thought there was a hint of green in the light).
As for not colouring the flame, I'm not sure. But there are a lot of metals that don't colour flames noticeably, even though they should give
interesting spectra. Maybe the flame just isn't hot enough?
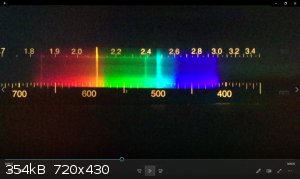
ETA: This is a spectrum I took of burning magnesium with a cheap plastic spectrometer (one step up from the paper/CD pocket version). Lots of
blackbody radiation, some sodium and lithium impurities, and a significant green line. I honestly don't know what that cyan line is- it's not a
magnesium emission, afaicfo.
[Edited on 24-1-2021 by DraconicAcid]
[Edited on 24-1-2021 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
| Quote: | | Salts of Ca, Sr, Ba color the flame resp. orange, red, yellowish green. |
Only in the presence of a chlorine donor. The colors come from respectively CaCl+, SrCl+and BaCl+. In fact, barium
and calcium metal burn with a bright white light, very similar to magnesium. (Strontium probably also does, I've never seen burning strontium though).
There are many pyrotechnics compositions containing barium nitrate or barium sulphate as the main oxidiser that emit bright white light because they
do not contain a chlorine donor.
You might ask what color MgCl+ emits, and I don't know. If it exists, and forms in fireworks flame conditions, it can't have a very
brightly coloured emissions spectrum because magnesium is the preferred metal fuel in coloured fireworks because its reaction products are more
volatile than those of aluminium, and therefore produce less black-body radiation that tends to wash out the color.
[Edited on 24-1-2021 by phlogiston]
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Probably a MgO band
https://www.sciencedirect.com/science/article/abs/pii/001021...
|
|
|
Bedlasky
International Hazard
    
Posts: 1244
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Phlogiston: I use strontium nitrate for making red flame. You don't need chloride for this.
|
|
|
Fulmen
International Hazard
    
Posts: 1726
Registered: 24-9-2005
Member Is Online
Mood: Bored
|
|
Some elements work in virtually any conditions, others need very specific conditions. The boiling point of the compound is one factor, as solids
produce back body radiation (continuous spectrum) while gaseous species produce narrow spectrum emissions. But since these narrow bands are produced
by electron excitation they are affected by chemical bonds.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
Bedlasky, that is interesting. Can you share the composition?
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
https://physics.nist.gov/PhysRefData/Handbook/Tables/magnesi...
tl;dr: the strong lines of Mg are actually in the UV at 280 and 285 nm, and these are more than ten times as strong as anything in the
visible spectrum. this is also part of the reason why burning Mg is so dangerous to your eyes.
oddly i've never heard of a UV lamp that uses this effect
|
|
|
Bedlasky
International Hazard
    
Posts: 1244
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Composition of what? I use just strontium nitrate.
|
|
|
xxxhibition
Harmless

Posts: 12
Registered: 25-1-2021
Member Is Offline
|
|
I found out a Mixture of 55% - 60% Strontiumnitrat and 45% - 40% Hexamin
gives you a deep red saturated Flame.

|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
Quote: Originally posted by DraconicAcid  | When magnesium burns, it's mostly blackbody radiation (although I've long thought there was a hint of green in the light).
As for not colouring the flame, I'm not sure. But there are a lot of metals that don't colour flames noticeably, even though they should give
interesting spectra. Maybe the flame just isn't hot enough?
ETA: This is a spectrum I took of burning magnesium with a cheap plastic spectrometer (one step up from the paper/CD pocket version). Lots of
blackbody radiation, some sodium and lithium impurities, and a significant green line. I honestly don't know what that cyan line is- it's not a
magnesium emission, afaicfo.
[Edited on 24-1-2021 by DraconicAcid]
|
I checked burning Mg ribbon again with my spectroscope and saw like you, beside the yellow Na-D line, indeed the green and turquoise lines as well,
and when the brightness faded, these two lines appeared better as the white blackbody radiation fainted.
[Edited on 2021-1-26 by metalresearcher]
|
|
|
Metallophile
Hazard to Self
 
Posts: 88
Registered: 23-3-2018
Member Is Offline
Mood: No Mood
|
|
The green and turquoise lines around 520 and 500nm look kind of like Titanium to me. It has a whole bunch of bright(ish) lines from 498-506nm.
https://physics.nist.gov/PhysRefData/Handbook/Tables/titaniu...
|
|
|
DraconicAcid
International Hazard
    
Posts: 4357
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
The brightest visible line from magnesium is at 518 nm, which is the green one.
https://physics.nist.gov/PhysRefData/Handbook/Tables/magnesi...
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|