| Pages:
1
2 |
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Urea
Urea- there's a lot there. It's hard to keep track of the possibilities. Are the auto-condensation products of urea beyond biuret explored in
energetics? Biuret is found. I can find very little on triuret, tetrauret and pentauret. Everything found seems unsubstantiated or unrelated to EM.
Ie. from agriculture patents, (which is interesting in itself), but other than DNB, there seems very little.
Is there any exploration of the later condensation forms of urea in energetics?
; err I mean, whatchu think?
[Edited on 4-1-2021 by Hey Buddy]
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Should ammonia be liberated out of this bitch or what?

|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
An awful lot would depend on what you were trying to accomplish.
|
|
|
j_sum1
|
Thread Moved
3-1-2021 at 21:01 |
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Great, maybe somebody in beginnings will know about tetra and pentauret?
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
You get isocyanuric acid and ammeline.
This is how isocyanuric acid is made commercially.
You can also get various other azines and tetrazole compounds as low level contaminants.
https://en.wikipedia.org/wiki/Cyanuric_acid
https://en.wikipedia.org/wiki/Ammeline
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Truly, apart from ammonia generation, hydrazine and urea nitrate, are there any collection of useful purposes for urea?
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
For the record, threads of a general nature that open without a citation more properly belong in beginnings. It does not change the readership. But it
does change the organisation of topics.
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@Hey Buddy; urea is a very useful compound in terms of being a starting material. Apart from buiret, isocyanuric acid an ammeline which are simply
polymeric degradation products you can also derivatize it eg -> urea nitrate -> nitrourea -> semicarbazide -> whole world of
semicarbazones and heterocyclics. Aliphatic amine + urea -> substituted ureas.
It can be used to prepare aromatic amines eg.; 4-nitrobromobenzene + urea = 4-nitroaniline; 2,4-dinitrochlorobenzene + urea; 2,4dinitroaniline and the
related reaction picric acid + urea = picramide (2,4,6-trinitroaniline)
Alkalis + dry urea give cyanamides or cyanate depending on conditions.
From biuret via nitrobiuret to aminobiuret (actually a hydrazine derivative) to tetrauret
I have prepared buiret many times and I can tell you that none of the simple heating methods work very well. The two best methods unfortunately are
very amateur unfriendly!! (one is via thionyl chloride and the other via direct chlorination of molten urea give yields >60%).
Aqueous lime water gives ammonia, Hoffmann's degradation give hydrazine etc. There is an entire monograph dedicated to the chemistry of urea.
If you want more specific help you will need to be a bit more specific on what you are trying to achieve. For instance are you simply trying to find
some interesting /useful chemistry for it because you have a lot of it?
|
|
|
artemov
Hazard to Others
  
Posts: 181
Registered: 22-8-2018
Member Is Offline
|
|
Quick aside.
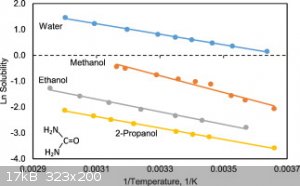
For the recrystallization/purification of urea, can water be used instead of ethanol/methanol?
The solubility curve of urea in water seems quite advantageous too ...
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Yes, boiling water can dissolve ~5 Kg/L while only ~.5 Kg/L will dissolve at 0 C.
edit: Urea is also great for electrolytic H2 production since it is extremely soluble in water and decomposes at ~.4 volts as opposed to water at ~1+
volts. As previously mentioned, it can be used to make cyanamides (heterocycle precursors, dehydrating agents, bases etc.) and isocyanates (also
heterocycle precursors and versatile reagents for the preparation of amines, carbamates, amides, carboxylic acids etc.). It is in my opinion one of
the most versatile and valuable reagents to the amateur since it can find so many uses at a very low cost.
[Edited on 1-4-2021 by njl]
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
artemov,
You need to compare the impurity and the desired chemical curves.
In the case of urea, the primary impurity is usually biuret which is more soluble and has a flatter curve in alcohols.
If you are purifying fertilizer the urea is often mixed with ammonium sulfate which is much less soluble in alcohol so it can be crashed out of a
water solution.
Ps. have you ever tried to pour or filter a saturated solution of urea in water?
Very viscous.
[Edited on 4-1-2021 by macckone]
|
|
|
artemov
Hazard to Others
  
Posts: 181
Registered: 22-8-2018
Member Is Offline
|
|
Thank njl and macckone.
Quote: Originally posted by macckone  |
If you are purifying fertilizer the urea is often mixed with ammonium sulfate which is much less soluble in alcohol so it can be crashed out of a
water solution.
[Edited on 4-1-2021 by macckone] |
I dun really understand this part. Ammonium sulfate is much less soluble in alcohol, but it can be crashed out of a water solution?
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Quote: Originally posted by j_sum1  |
For the record, threads of a general nature that open without a citation more properly belong in beginnings. It does not change the readership. But it
does change the organisation of topics. |
Thanks for mentioning this. I thought I was being taken out backdoor by the ear, maybe I peed on a rug or something.
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Quote: Originally posted by Boffis  | @Hey Buddy; urea is a very useful compound in terms of being a starting material. Apart from buiret, isocyanuric acid an ammeline which are simply
polymeric degradation products you can also derivatize it eg -> urea nitrate -> nitrourea -> semicarbazide -> whole world of
semicarbazones and heterocyclics. Aliphatic amine + urea -> substituted ureas.
It can be used to prepare aromatic amines eg.; 4-nitrobromobenzene + urea = 4-nitroaniline; 2,4-dinitrochlorobenzene + urea; 2,4dinitroaniline and the
related reaction picric acid + urea = picramide (2,4,6-trinitroaniline)
Alkalis + dry urea give cyanamides or cyanate depending on conditions.
From biuret via nitrobiuret to aminobiuret (actually a hydrazine derivative) to tetrauret
I have prepared buiret many times and I can tell you that none of the simple heating methods work very well. The two best methods unfortunately are
very amateur unfriendly!! (one is via thionyl chloride and the other via direct chlorination of molten urea give yields >60%).
Aqueous lime water gives ammonia, Hoffmann's degradation give hydrazine etc. There is an entire monograph dedicated to the chemistry of urea.
If you want more specific help you will need to be a bit more specific on what you are trying to achieve. For instance are you simply trying to find
some interesting /useful chemistry for it because you have a lot of it? |
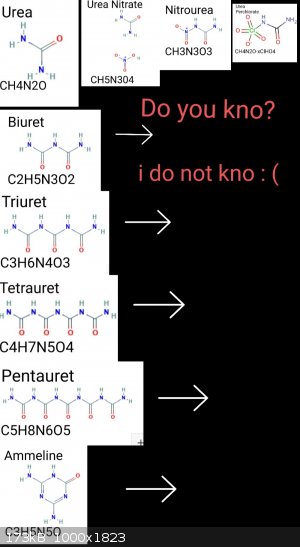
This is what I've come to understand. And correct me with anything I'm not understanding; That urea is both a simple molecule and a functional
group(?). Massive exponential spread of possible extensions in step series. > GN, DPT, Hydrazine, semicarbazide, carbohydrazone, NH3, etc.
In terms of energetics, upon heating under specific conditions,, a series of condensation transformations occur. The transition is not a linear
progression by temp, but by a heating and pressure/oxygen matter. Have the macromolecules been explored? I can't find anything.
Attachment: US3453098.pdf (529kB)
This file has been downloaded 287 times


It's all quiet now. Maybe I've asked something stupid?
Is there any reason not to nitrate or perchlorate the higher condensation products or macomolecules in general? I'm totally unfamiliar with this, and
chemistry in general, so I don't know if this is a silly question. Any help?
[Edited on 9-1-2021 by Hey Buddy]
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Update: I've found energetic research on melamine, ammeline, and cyuranic. Of course biuret is already widely explored..
Have still not located any research on triuret tetrauret or pentauret, which are all condensation products. If anyone is aware of research on
energetic compounds of those please respond.
Have located patents on isolation processes for triuret but nothing on tetrauret nor penta. If anyone knows a process to isolate those please
respond.
Id like to experiment with urea beginning with the lesser explored areas first, thank you.
[Edited on 14-1-2021 by Hey Buddy]
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
In Boffis' post above they describe the route to isolate tetrauret.
There is more information in here, also provided by Boffis under another thread.
Very interesting stuff!
http://www.sciencemadness.org/talk/files.php?pid=613206&...
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Excellent, thank you. --Two documents I've found of interest are a 1950s procedure of melamine and other products by simple heating in pipes at
various densities and Temps. The second is experimental tetrauret macrocycles. Really a dizzying array of possibilities with urea. It's plastic
derivatives are fascinating too. Apparently urea products can be used for desensitization coating of classics like RDX.
Attachment: Melamine from Urea.pdf (1.8MB)
This file has been downloaded 450 times
Attachment: Tetraurea.pdf (2.7MB)
This file has been downloaded 395 times
[Edited on 16-1-2021 by Hey Buddy]
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
The urea likes to recrystalyze

|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Update: Have worked up through biuret to melamine.
Experimenting now with gCN/BgCN. Biuret is very easy in isolation, as it's the first condensation, limited to temp ceiling 189C. Biuret conversion
appears reasonsbly possible in open vessels. Triuret, tetrauret, pentauret, cyuranic, ammelene, ammelide, are much more difficult in discrimination.
The isolation of these by thermal condensation without gas feed is not well understood. building some vessels for pyrolytic condensation and gas feed
now.
Currently in digressions of gCN. Sort of necessary pit stops on the way to EMs. Its watersplitting ability in solar applications and the gCN catalytic
abilities, which seem pretty incredible. Within this there is microwave production of melem using KNO3 as an exfoliator. Urea and KNO3 mixes produce
high surface area gCN for cats. But I'm interested in its effect on homogenous formation of melamine enroute to melem.
The most significant condensation of urea where EM is concerned, (imo) is melem/melon. The production of gCN passes through the stages of melamine and
then melem prior to being calcinated into gCN.
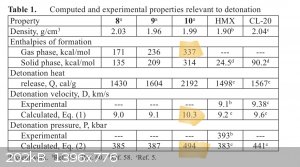
Melem is a heptazine from urea. Melon appears to be an autocondensed poly lattice of melem. Energetic heptazines and be made from melem. It is pretty
unexplored for the most part. A lot of theoretical compounds. I've read the claim that n-oxide impinged heptazines are theorized to have impact and
thermal sensitivity from the range of TNT all the way up to RDX with several of the compounds having greater vod than cl20. Most of the theoretical
compounds have greater vod than HMX and with less sensitivity. This is the reason i am interested in Urea. Its products are a vast library and in this
area it can be purchased bulk by anyone at $316/Ton.
Will continue to explore and post useful findings. see attachments on heptazines.
I dont schpreken ze Deutsch is anyone would like to help explain that 2nd one i would love to know more. Thank you.
Attachment: Heptazine n-oxide high performance.pdf (1.5MB)
This file has been downloaded 333 times
Attachment: Deutsch _hepta.pdf (474kB)
This file has been downloaded 316 times
 
[Edited on 26-1-2021 by Hey Buddy]
[Edited on 26-1-2021 by Hey Buddy]

|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Forgive my ignorance but is gCN/BgCN cyanoguanidine and cyanobiguanidine (correct nomenclature aside)? When you say "kno3 and urea produce high
surface area for cats", do you mean for catalysis?
|
|
|
rockyit98
Hazard to Others
  
Posts: 283
Registered: 12-4-2019
Location: The Known Universe
Member Is Offline
Mood: no mood is a good mood
|
|
Making Graphitic carbon nitride (g-C3N4) from urea
https://www.sciencemadness.org/whisper/viewthread.php?tid=154866#pid631050
"A mind is a terrible thing to lose"-Meisner
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
Quote: Originally posted by njl  | | Forgive my ignorance but is gCN/BgCN cyanoguanidine and cyanobiguanidine (correct nomenclature aside)? When you say "kno3 and urea produce high
surface area for cats", do you mean for catalysis? |
My apologies, "gCxNx" is the way graphitic carbon nitride is described in energy study literature. BgCN is boron graphene carbon nitride. "Cat" im
just contracting "catalyst".
Here is some info on KNO3/Urea catalysts look under "CNU-30":
Attachment: Microwave melamine carbon nitride.pdf (6.9MB)
This file has been downloaded 390 times
|
|
|
Hey Buddy
Hazard to Others
  
Posts: 441
Registered: 3-11-2020
Location: Bushwhacker Country
Member Is Offline
|
|
See what I'm talkin about?
Urea.
It's where it's at.
Urea covers everything from low order det to fastest and everything between.
Attachment: Polyazine.pdf (791kB)
This file has been downloaded 300 times
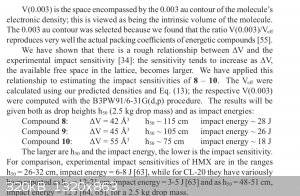

|
|
|
RustyShackleford
Hazard to Others
  
Posts: 200
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
Very much is, i did it in a 500ml beaker with a RBF on top. worked perfectly fine. The isolation of the biuret is easier with beaker also, just add
dilute hydroxide (2-3% or so, as long as it stays above 9-10) , heat till dissolved, cool and collect crystals, then rex it from distilled water to
rid the last trace of hydroxide.
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
artemov,
Crashing out of water using alcohol as a cosolvent is useful for a number of purifications.
The basic procedure is to dissolve a urea/ammonium sulfate mixture in minimal water.
Then add about 4 times as much dry alcohol as solution.
The ammonium sulfate will form fine crystals free from urea.
Ammonium sulfate is only slightly soluble in 80% alcohol while urea is very soluble.
The procedure I actually use involves dissolving it in significantly more water than necessary and evaporating most of it off, then adding alcohol.
Since the stuff I get is fertilizer grade, I can filter off insoluble impurities with the excess water.
Then get nice pure crystals that I can rinse with alcohol to get rid of any remaining urea.
The alcohol can be redistilled and reused.
The remaining urea can be recrystalized from dry alcohol.
The mix I get is about 90% ammonium sulfate and 10% urea.
|
|
|
| Pages:
1
2 |