EverythingAl2O3
Hazard to Self
 
Posts: 51
Registered: 3-9-2019
Member Is Offline
|
|
An attempt at making Phorone
My first attempt at making phorone. I plan on using it to try and synthesize y-halo ketones/aldehydes. I'm going to attempt to use the CuO, I2 method
to see if that halogenation method works in a vinylogous manner (It has already been put on another post but I'll post it below). If it doesn't then I
guess I'll try to figure something else out because I can't find any papers on y-halo ketone synthesis.
I started with 150mL Acetone and 150mL HCl and left it to react for 2 hours 35 minutes (at 40-50C) because I have no idea how long it needs to run
and the color appeared to stop changing after that point.

15 minutes in I got a slight yellow color that looks like the wikipedia description of phorone

40 minutes

88 minutes

124 minutes

155 minutes I decided to neutralize the acid with NaHCO3. Unfortunately NaCl precipitated out and I had to gravity filter the solution, but a lot
remained trapped in the salt slurry. The remaining solution was then neutralized and once no more CO2 came of the solution went from red to clear with
yellow oil drops that separated into a distinct later from the aqueous solution (I did not take pictures of this part). I have not finished working
with the salt slurry but I did get a very small amount of what I believe is phorone.

I think this would work better if I distilled off the acetone, HCl and mesityl oxide and then add a small portion of acetone to the distillate to
convert the mesityl oxide to phorone. Then with a significantly smaller volume of liquid I can remove any remaining HCl in the phorone. The current
yield is too low to begin calculating the yield.
Attachment: CuO ketone iodination.pdf (105kB)
This file has been downloaded 485 times
[Edited on 13-1-2021 by EverythingAl2O3]
|
|
|
mr_bovinejony
Hazard to Others
  
Posts: 130
Registered: 20-4-2018
Member Is Offline
Mood: ASS
|
|
Very cool! This can be used as a precursor for TEMPO which is a very useful oxidizing catalyst. There's a thread around here about it I think
|
|
|
EverythingAl2O3
Hazard to Self
 
Posts: 51
Registered: 3-9-2019
Member Is Offline
|
|
| Quote: |
Very cool! This can be used as a precursor for TEMPO which is a very useful oxidizing catalyst. There's a thread around here about it I think
|
I have seen that and think TEMPO is a really cool reagent and might use any leftover phorone to make some although I'm not sure how well I will be
able to employ it. I want to play with vinylogy and Al2O3 catalyzed reactions and TEMPO synthesis from phorone looks like a vinylogous type reaction
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Hi EverythingAl2O3, you did very nice experiment! I found this article about synthesis, I hope it could be helpful to you.
http://npcomplete-solutions.com/Projects/Research/PhoroneSyn...
and this link here in the forum:
http://www.sciencemadness.org/talk/viewthread.php?tid=1203
|
|
|
EverythingAl2O3
Hazard to Self
 
Posts: 51
Registered: 3-9-2019
Member Is Offline
|
|
Thanks. I have already read both of those, but I did not have the equipment to generate dry HCl and I do not have AlCl3 so I did simple 33% HCl
because I know Aldol doesn't care too much about water. I will be trying to make some in the future following an interesting patent I found that
claims anhydrous AlCl3 can be made from saturated aqueous AlCl3 and the addition of an alcohol.
|
|
|
EverythingAl2O3
Hazard to Self
 
Posts: 51
Registered: 3-9-2019
Member Is Offline
|
|
Attempt 2 update
I redid the reaction using 100mL of acetone and 100mL 33% HCl and left it overnight at 50C. After stirring stopped many oil drops formed on the
surface of the solution. I have added an additional 30mL of acetone to try and convert more mesityl oxide to phorone. We will see how that goes in a
few hours

|
|
|
EverythingAl2O3
Hazard to Self
 
Posts: 51
Registered: 3-9-2019
Member Is Offline
|
|
Phorone update 2 product after acetone addition
So...This did not go anything like it did previously. I added an additional 30mL of acetone after reacting overnight, and left if for 5 hours. Upon
neutralization instead of getting a thin yellow oil layer developing and all the red coloration disappearing, I have been greeted with this deep band
of dark red oil on top of the water layer (with some phorone dissolved in) which probably only separated because NaCl salted it out. So not sure how
much mesityl oxide is in this layer or if this is pure phorone which since it is more concentrated has altered appearances. I guess simple
distillation or recrystallization will determine that? What I do know is that all the acetone has been consumed because at 75C no boiling began unlike
my first run.

|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi EverythingAl2O3, I've been following your experimental endeavors with interest as I have been contemplating attempting this reaction also. Have you
seen this paper:
Attachment: Preparation of Phorone and Mesitylene oxide ZNatB Konieczny & Sosnovsky 1978.pdf (6.8MB)
This file has been downloaded 493 times
Its a pretty comprehensive examination of your reaction. Good luck and keep up the good work! Oh and remember to keep us informed 
|
|
|
kmno4
International Hazard
    
Posts: 1504
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Acid- or base* catalyzed condensation of acetone may give (and often gives) c.a. 20 different products at the same time, with yields depending on
everything. There is a thread on the board, strarted by Polverone(?) about promissing phorone preparation (some old article based, I think so).
* of course, in some very few cases, like diacetone alcohol, the reaction can be forced in practically on direction.
Слава Україні !
Героям слава !
|
|
|
EverythingAl2O3
Hazard to Self
 
Posts: 51
Registered: 3-9-2019
Member Is Offline
|
|
| Quote: |
Hi EverythingAl2O3, I've been following your experimental endeavors with interest as I have been contemplating attempting this reaction also. Have you
seen this paper:
Its a pretty comprehensive examination of your reaction. Good luck and keep up the good work! Oh and remember to keep us informed 
|
I actually did come across it in researchgate, but the AlCl3 is the issue. I do not have access to anhydrous HCl or anhydrous AlCl3. I might have
found a patent that can solve the AlCl3 issue (will link and paste an excerpt) but I still can't generate anhydrous HCl due to lack of equipment at the moment.
However looking at this paper again, it makes me hopeful that I can solve a few issues that I may have over looked and make way for new experiments. I
don't think I have the any chloro products because of how I treated the product during workup. I left it overnight in a basic solution so if anything
I have an alcohol group on the tertiary carbon so NaOH should be able to eliminate it to phorone if this is the case.
AlCl3's behavior might help me with a more selective cross aldol and do another few tests for something else I'm working on with beta-haloketones.
A following reaction that I was going to perform was a reduction with dithionite to see if it will produce an alcohol or reduce the halogen instead
like with alpha haloketones.
Here is an example
Taking 10 g of aluminum chloride hexahydrate, putting the aluminum chloride hexahydrate into a beaker, adding water to prepare an aqueous solution,
filtering to remove impurities, slowly pouring commercially available methanol, and after crystals in the beaker are fully precipitated, carrying out
solid-liquid separation in a centrifugal separator to obtain white crystals, namely the anhydrous aluminum chloride. The solid-liquid separation
according to the above method can also be carried out by other methods such as ordinary filtration. Or filtering to remove impurities after preparing
the aluminum chloride hexahydrate into an aqueous solution.
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I hate to say this but I think that chinese patent is garbage. Just look at how much energy is released when aluminium chloride is hydrated, I can't
believe the water can be displaced with a small amount of low molecular weight alcohol. I doubt that you could remove the water by azeotropic
distillation with vast amounts of absolute ethanol. Fairy tale stuff
|
|
|
EverythingAl2O3
Hazard to Self
 
Posts: 51
Registered: 3-9-2019
Member Is Offline
|
|
Honestly did think it was too good to be true but thought it wouldn't hurt to try anyway
|
|
|
EverythingAl2O3
Hazard to Self
 
Posts: 51
Registered: 3-9-2019
Member Is Offline
|
|
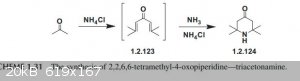
Possible alternative pathway to phorone
I found this paper in the link that shows the synthesis of triacetoneamine, but the scheme shows phorone as an intermediate, almost like NH4Cl can
directly catalyze the synthesis of phorone and the addition of NH3 then forms the amine. I did a little more digging on NH4Cl aldol condensation and
found a paper that shows the reaction of aromatic aldehydes and cyclopentanone forming the desired double aldol condensation. I will be investigating
this process next as soon as I get some urea to make NH4Cl.
https://sci-hub.se/https://doi.org/10.1016/B978-0-12-805157-...
Attachment: Ammonium chloride aldol.pdf (209kB)
This file has been downloaded 420 times
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Hi EverythingAl2O3 - nice findings, from the link you posted I extracted the necessary info for the triacetoneammine, quite complex apparatus required

https://sci-hub.st/10.1007/BF00760847
Attachment: rozantsev1971.pdf (334kB)
This file has been downloaded 402 times
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Regarding triacetonamine, you don't need such a complex apparatus if you're willing to tolerate lower yields. Back in the TEMPO challenge thread, I
made it by bubbling ammonia gas through a solution of acetone / methanol / NH4Cl
http://www.sciencemadness.org/talk/viewthread.php?tid=62358
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Hi Metacelsus, thx for the info and link!
I remembered that when buying from one ebay seller I saw something similar at reasonable price, but it was 4-hydroxy-TEMPO, I was able to find it
again:
https://www.ebay.com/itm/100g-4-Hidroxy-TEMPO-TEMPOL-flakes-...
|
|
|
EverythingAl2O3
Hazard to Self
 
Posts: 51
Registered: 3-9-2019
Member Is Offline
|
|
| Quote: |
Regarding triacetonamine, you don't need such a complex apparatus if you're willing to tolerate lower yields. Back in the TEMPO challenge thread, I
made it by bubbling ammonia gas through a solution of acetone / methanol / NH4Cl
|
Would phorone as a starting point instead of acetone improve yield? Also what causes the decrease in yield with typical bubbling of acetone?
Now I'm really interested in triacetone amine. It seems like an easily accessible organic base for things like kornblum oxidation, deprotonation of
dicarbonyls, etc.
|
|
|
EverythingAl2O3
Hazard to Self
 
Posts: 51
Registered: 3-9-2019
Member Is Offline
|
|
I have finally gotten some NH4Cl and I will be testing the phorone synthesis next break I get from school

|
|
|
kmno4
International Hazard
    
Posts: 1504
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
If someone likes acetone way to phorone, I would suggest condensation by NaOH or KOH, instead of HCl. Besides, it is important to realize that using
HC(aq) instead of HCl(g) is highly harmful for the reaction, because of very large amount of introduced water. Hydroxides-way is simpler and by-passes
problems with water.
Some info can be found here:
Aldol condensation of acetone in the two-phase system solid base-benzene in the presence of benzyltriethylammonium chloride
(DOI: 10.1007/BF00954072)
They use PTC catalyst, but it works also without it. Benzene is used for measurements of kinetics of the reaction, it can be replaced with any inert
solvent or simply excluded from the reaction.
Worth trying and playing with cheap reagents 
PS. Just for fun - experiment is started : 20 g of acetone + 16 g of NaOH (two independent runs, with better (fresh) and worse (old) hydroxide).
Because chemical reactions take pace only on the surface of the solid NaOH, it is important to use mag. stirring, or at least shaking from time to
time.
After ~12 h, the mixture becomes reddish and under acetone odour some other odour appears, reminding me methyl isobutyl ketone (MIK).
[Edited on 14-2-2021 by kmno4]
Слава Україні !
Героям слава !
|
|
|
EverythingAl2O3
Hazard to Self
 
Posts: 51
Registered: 3-9-2019
Member Is Offline
|
|
| Quote: |
If someone likes acetone way to phorone, I would suggest condensation by NaOH or KOH, instead of HCl. Besides, it is important to realize that using
HC(aq) instead of HCl(g) is highly harmful for the reaction, because of very large amount of introduced water. Hydroxides-way is simpler and by-passes
problems with water.
Some info can be found here:
Aldol condensation of acetone in the two-phase system solid base-benzene in the presence of benzyltriethylammonium chloride
(DOI: 10.1007/BF00954072)
They use PTC catalyst, but it works also without it. Benzene is used for measurements of kinetics of the reaction, it can be replaced with any inert
solvent or simply excluded from the reaction.
Worth trying and playing with cheap reagents 
PS. Just for fun - experiment is started : 20 g of acetone + 16 g of NaOH (two independent runs, with better (fresh) and worse (old) hydroxide).
Because chemical reactions take pace only on the surface of the solid NaOH, it is important to use mag. stirring, or at least shaking from time to
time.
After ~12 h, the mixture becomes reddish and under acetone odour some other odour appears, reminding me methyl isobutyl ketone (MIK).
|
I considered NaOH but am probably overly concerned with glassware damage. I will try that next after I have finished NH4Cl testing
|
|
|
kmno4
International Hazard
    
Posts: 1504
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Acetone + NaOH : some observations.
First - the reaction is very slow, even added PTC catalyst (TBACl) does not improve conversion of acetone. No more than 20 % conversion was obtained
after ~48 hours. It was estimated by evaporation a sample on watch glass, when oily residue, without acetone odour was obtained. The odour of this oil
is interesting and remids me camphor.
Second - large amount of solids is produced during reaction as suspension and quickly it becomes impossible to stirr it magnetically. The most
possibly, the solid is just NaOH with absorbed (solvated ?) some primary product of condensation (diacetone alcohol (DA) ?). Something like this is
observed in the mentioned paper - quick disappearance of formed DA trom the solution.
The amout NaOH given above is - probably -, far too much.
Experiment with ~4 g of NaOH (quickly powdered in a mortar) gave very similar effects.
Who knows, maybe ~50% water solution of NaOH would be better ?
Something to read before starting experiments with ammonia+acetone:
Towards a more sustainable production of triacetoneamine with heterogeneous catalysis (DOI:10.1016/j.molcata.2014.06.023)
It is minireview (from 2014) and seems to be rather reliable paper, comparing to bullshit patent lierature or related rubbish.
Triacetoneamine is cheap compound to buy, but not so easy to make "at home". However, as far as I remember, Klute succeeded.
[Edited on 16-2-2021 by kmno4]
Слава Україні !
Героям слава !
|
|
|
kmno4
International Hazard
    
Posts: 1504
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
The fun goes on - to the mixture of acetone and NaOH, some heptane was added (10 cm3 per 40 g of acetone), water was added in such amount to dissolve
almost all NaOH. Separate layers are formed, after additional time (stirring, stirring....) conversion of acetone into oily products reaches ~30%.
I will try to obtain at least 50% of conversion, and then try to do something with the oil.
Слава Україні !
Героям слава !
|
|
|
kmno4
International Hazard
    
Posts: 1504
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
No progress is obtained with saturated NaOH solution.
The conversion of acetone cannot be increased beyond ~20-30%, at least in my hands. Distilling off all volaltiles and performing steam dist. of
residue, small amout of yellowish, clean oil (density less than water) is obtained. It has interesting odour, resembling camphor/turpentine, with
distant echos of phenylacetone and cat's urine. The amount is too smal to perform normal distillation.
Certaily it is a mixture of many compounds, known as "(iso)xylitones". The residue from steam dist. is brown, sticky resin.
I tried KOH, instead of NaOH (just for fun) and it turns out much more reactive than the latter. When KOH is added to acetone, the mixture becomes
warm and remains warm for longer time. The colour of solution becomes red during few minutes and some solid satarts to collect. Unfortunately, it
seems that there is not much improvement in acetone condensation, but it is still under investigation.
Few comments to the article I mentioned.
1) the autors use word "acetonie" but in literature "acetonin" is much more common.
2) a compound, described there as "2,2,4,6-tetramethyl-2,5-dihydropyridine" (TMDP), should be named correctly as
2,2,4,6-tetramethyl-2,3-dihydropyridine.
By the way, it is said that TMDP turns to brown resin in the air.
It explains, at last, why in all my earlier experiments with acetone/ammonia, I got such intractable resin.
Слава Україні !
Героям слава !
|
|
|
EverythingAl2O3
Hazard to Self
 
Posts: 51
Registered: 3-9-2019
Member Is Offline
|
|
NH4Cl test
So for my initial NH4Cl test I started with 28g NH4Cl, and 100mL Acetone with 100mL water co-solvent to help increase direct contact of NH4Cl with the
acetone and this was done at room temp. The clear image is after 24 hours and the yellow image is after 3 weeks sitting at around 5C in a cold
basement. Looking back the water was probably unnecessary with strong stirring, but it is probably the reason why I saw results leaving it to sit.
Other issues the may have played into the slow reaction time is the particle size of NH4Cl and from a paper I listed above in the thread NH4Cl
catalyzed reactions showed no signs of self aldol even after 5 hours at reflux temps in ethanol so maybe this is just a really reluctant reaction.
Granted this is in the presence of a competing nucleophile so that may have altered the behavior. But it seems that it does eventually work.
The next plan is to increase the heating to 50C for 24 hours and test water co-solvent and solventless to see if a solvent is necessary with a liquid
 
|
|
|
EverythingAl2O3
Hazard to Self
 
Posts: 51
Registered: 3-9-2019
Member Is Offline
|
|
Just an update on the phorone tests. One the left image we see acetone with NH4Cl with no solvent after 2 months. No significant color change except a
slight yellow tinge once all the NH4Cl settles out. On the right we have the same test of phorone with NH4Cl as previous updates. It has progressed to
a dark red-brown with yellow at the edges of the liquid. There are no oil drops on the surface of the solution unlike with the HCl tests, so I do not
know if I have the right product here. The solution gives off a sweet, honey-like odor which is like dilute acetone, but maybe it is mesityl oxide? I
currently do not have time to separate the products so I don't know what the yield is like.
As a side note, the time that it has taken for the color change to occur makes me hopeful for using NH4Cl to prevent self aldol in other aldol
condensation reactions. The paper that noticed this occurrence is in a previous update I made.
 
|
|
|