Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
hydrobenzamide from benzaldehyde and ammonia
Everyone knows what is the product of reaction of formaldehyde with aqueous ammonia. Hexamethylenetetraamine.
https://en.wikipedia.org/wiki/Hexamethylenetetramine
Reactants in ratio 6 aldehyde + 4 ammonia. Nice regular space structured molecule.
Less people know what is the product of reaction of acetaldehyde with aqeous ammonia. It is Acetaldehyde ammonia trimer
https://en.wikipedia.org/wiki/Acetaldehyde_ammonia_trimer
Reactants in ratio 3 aldehyde + 3 ammonia. Nice planar ring molecule.
Even less people know what is the product of reaction of benzaldehyde with aqueous ammonia. It is hydrobenzamide. Molecule not so much symmetrically
shaped as the previous 2. But useful in preparation of further compounds. There is also a possibility to form a trimer product in certain solvents
(see lozynskaya2003.pdf or the corresponding link, look there for "scheme 2").
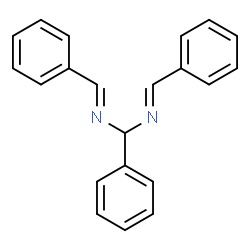
Enough of words, let's do something and investigate it practically!
Usefull info grabbed over internet:
https://prepchem.com/synthesis-of-hydrobenzamide/
https://illumina-chemie.de/viewtopic.php?t=3657
10,5g benzaldehyde + 50 ml 25% NH3 magnetically stirred at highest possible RPM for 2 hours in 50 ml Erlenmayer flask which neck airtightly closed
with plastic sheet (so only very little of air present), then let to stay for 3 days. Hard product adhering to the bottom formed, solution of remained
ammonia poured out and the product broken to smaller pieces with thick glass rod.
Crystalized from 96% ethanol (free of denaturing agents like denatonium benzoate because I want to taste 1 of its derivates which should taste
bitter). On the first recrystallization from 50 ml of ethanol an oil separated which later solidified, so I decided to recrystallize it again from 50
ml ethanol when nothing crystallized after hours, but I put it into freezer at -18 C for 1 week and then the product crystallized. Filtered and air
dried on filter paper. Still some decent scent resembling benzaldehyd present. Perhaps it decomposes back into reactants in the 96% ethanol on
heating? I did not use freshly distilled benzaldehyde as reactant so maybe the first crystallization when oil separated was caused by some benzoic
acid contamination?
yield 5,3 g (cca 50%, not too much perhaps due the necessity of 2 crystallizations? or contamination with benzoic acid so less benzaldehyde reacted =
oxidation with air oxygen which destroys benzaldehyde?)
m.p. 100-103 C
    
Now what it could be used for:
hydrobenzamide -> amarine -> lophine (weak chemiluminiscent)
hydrobenzamide -> amarine -> isoamarine -> stilbenediamine (investigating complexes, especially with Ni see the attached corey1997.pdf or the
link, Co see williams1959.pdf or the link, also see lifschitz1940.pdf or the link)
http://www.chemspider.com/Chemical-Structure.7841195.html
melting point:
102-105 °C Alfa Aesar
104 °C Jean-Claude Bradley Open Melting Point Dataset 7312
102-105 °C Alfa Aesar H53492
102-105 °C Sigma-Aldrich ALDRICH-167649
https://books.google.cz/books?id=jgZLDwAAQBAJ&pg=PA679&a...
679.png
https://books.google.cz/books?id=MAvDDwAAQBAJ&pg=PT92&am...
toxicity.png
https://physoc.onlinelibrary.wiley.com/doi/pdf/10.1113/jphys...
https://ru.wikipedia.org/wiki/%D0%90%D0%BC%D0%B0%D1%80%D0%B8...
amarin.zip
https://sci-hub.st/10.1002/cber.188001301197
fisher1880.pdf
https://youtu.be/8XOKsHRp-f0
lophine from benzil + benzaldehyde + NH3HCO3 + acetic acid
https://sci-hub.st/10.1021/ed083p1658
crouch2006.pdf
from benzaldehyde and benzil in microwave
https://www.nature.com/articles/s41467-019-08816-8
https://www.nature.com/articles/s41467-019-08816-8.pdf
https://static-content.springer.com/esm/art%3A10.1038%2Fs414...
lophine hydroperoxide synthesis
https://sci-hub.st/10.1039/C4PP00311J
alves2015.pdf
chemiluminiscence mechanism
https://prepchem.com/synthesis-of-hydrobenzamide/
Preparation of hydrobenzamide
5 ml of benzaldehyde and 25 ml conc. ammonium hydroxide solution are placed in a stoppered flask and allowed to stand for 2 days. Crystals of
hydrobenzamide separate, which are filtered off, washed with water, and recrystallised from alcohol. Yield 90% theoretical (4.2 gms.). m.p. 110°
C; insoluble in water, easily soluble in alcohol.
Systematic organic chemistry, by W. M. Cumming, 307, 1937.
https://sci-hub.st/10.1023/A:1023915024572
lozinskaya2003.pdf
https://www.sigmaaldrich.com/catalog/product/aldrich/167649?...
A simplified synthesis of (?)-1, 2-diphenyl-1, 2-diaminoethane (1) from benzaldehyde and ammonia. Revision of the structures of the long-known
intermediates ?hydrobenzamide? and ?amarine?. Corey EJ and Kuhnle FNM. Tetrahedron Letters 38(50), 8631-8634, (1997)
Synthesis and solution studies of Cu (II) complexes with pyridine derivatives of iminobisphosphonic acids. Goldeman W, et al. Inorgorganica Chimica
Acta 365(1), 391-399, (2011)
https://sci-hub.st/10.1016/S0040-4039(97)10372-0
corey1997.pdf
https://sci-hub.st/10.1021/ja01526a007
williams1959.pdf
https://sci-hub.st/10.1002/recl.19400590208
lifschitz1940.pdf
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
sources downloaded for the case they disappear

Attachment: 5105-Article Text-11073-1-10-20130301.pdf (451kB)
This file has been downloaded 362 times
Attachment: 41467_2019_8816_MOESM1_ESM.pdf (1.7MB)
This file has been downloaded 425 times
Attachment: alves2015.pdf (369kB)
This file has been downloaded 382 times
Attachment: amarin.zip (95kB)
This file has been downloaded 350 times
Attachment: corey1997.pdf (195kB)
This file has been downloaded 395 times
Attachment: crouch2006.pdf (105kB)
This file has been downloaded 378 times
Attachment: fischer1880.pdf (307kB)
This file has been downloaded 330 times
Attachment: html.zip (23kB)
This file has been downloaded 335 times
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
continuation of sources downloaded for the case they disappear
Attachment: jphysiol.1948.sp004276.pdf (2.4MB)
This file has been downloaded 1525 times
Attachment: lifschitz1940.pdf (561kB)
This file has been downloaded 376 times
Attachment: lozinskaya2003.pdf (342kB)
This file has been downloaded 362 times
Attachment: s41467-019-08816-8.pdf (2.4MB)
This file has been downloaded 440 times 
Attachment: williams1959.pdf (922kB)
This file has been downloaded 373 times
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Nice work Fery and really well referenced. There are some interesting sounding derivatives too.
I prepared some lophine a few years ago as part of my investigation of thiamine catalyzed benzoin formation followed by oxidation to benzil and then
reacted this with benzaldehyde and ammonia. I wounder if you can produce amarine by carrying out the reaction with benzoin?
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Excellent write up!
|
|
|
DraconicAcid
International Hazard
    
Posts: 4356
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I wonder if it's big enough to be a bidentate ligand....
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Hi Boffis, such a trivial reaction in only the most basal glassware and till yesterday not yet referenced here in sciencemadness forum.
Your route is referenced in my first post and you can download the attached files also, I had to split everything into 3 posts as it failed approx 5
times to post everything together in 1 post:
https://youtu.be/8XOKsHRp-f0
lophine from benzil + benzaldehyde + NH3HCO3 + acetic acid
https://sci-hub.st/10.1021/ed083p1658
crouch2006.pdf
from benzaldehyde and benzil in microwave
I already started preparation using ethanol solution of NH3 instead of 25% water solution with a hope of higher yield and purer product, because
Polish chemist Bronislav Radzishevsky (inventor of this reaction) did it that way, as it is referenced in the Russian wiki mentioned in the first post
https://ru.wikipedia.org/wiki/%D0%90%D0%BC%D0%B0%D1%80%D0%B8...
and I also downloaded the page and packed into file amarin.zip available for download with a machine translation into eng (I still understand Russian
language but not so well as in elementary school due to not practicing for decades).
DraconicAcid:
look into the links referenced in the 1st post or download the attached files from one of the following posts:
Cobalt complexes
https://sci-hub.st/10.1021/ja01526a007
williams1959.pdf
Nickel complex (it is in Deutsche Sprache - Die Nickelkomplexe) - again referenced in my first post and download available in one of the following
posts
https://sci-hub.st/10.1002/recl.19400590208
lifschitz1940.pdf
|
|
|
DraconicAcid
International Hazard
    
Posts: 4356
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I think in these papers, they are using the hydrobenzamide to make stilbenediamine, which they then make complexes from. I'm not seeing direct
complexes of hydrobenzamide.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
DraconicAcid - hydrobenzamide is insoluble in water.
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
synthesis in ethanol environment
I prepared NH3 in ethanol:
Absorbtion bottle with sintered tube was filled with 40,0 g 96% ethanol using syringe and needle as this otherwise powerful absorbtion bottle has a
disadvantage that it cannot be disassembled. Commercial denatured ethanol refluxed with NaOH to get rid of 1,6% MEK and distilled to get rid of
denatonium benzoate was used.
The NH3 generator was a 250 ml flask which was charged with 0,5 mol (NH4)2SO4. 1 mol NaOH was dissolved in 50 ml H2O in a beaker while cooling in cold
water bath. The NaOH solution was added at once to the generator and the flask was quickly closed with well greased take-off adapter connected with 2
m long hose which other end was connected to the absorbtion bottle. The gas was driven slightly upwards so eventual H2O vapor condensation could not
drop into the absorbtion bottle. It was done outside of building at temperature 0 C so the hose served also as an condenser. The NH3 evolution started
immediately and the flask was shaken in hand and later heated in hot oil bath until the evolution of NH3 ceased. The weight increase of the absorbtion
bottle showed that the NH3 concentration was 14 %. Later I charged the same flask with 0,25 mole of (NH4)2SO4 and added to it 0,5 mol of solid NaOH
and then added 5 ml of water to start the reaction. The flask was again shaken and heated in hot oil bath until the evolution of NH3 finished.
Weight difference of the absorbtion bottle showed that 7,8 g NH3 absorbed totally in 40,0 g of ethanol at the end of second gassing. Both gassings
together lasted slightly more than 1 hour. The absorbtion bottle was cooled by cold surrounding air (actual outside temperature 0 C) as the absorbtion
was exothermic. So final NH3 weight concentration was 16%. There was also an increase in volume (the black mark on the absorbtion bottle was original
volume.
    
10,6 g benzaldehyde (100 mmol, M=106,12 g/mol) + 7,8 g NH3 in 40 g of ethanol mixed in 100 ml Erlenmayer flask, closed with plastic sheet and rubber
band (to reduce banzaldehyde oxydation by air oxygen as much as possible) and placed outside for the night (outside temperature 0 C) as the gas
evolution immediately visible on the plastic which was expanding like a bubble. Nothing crystallized overnight at 0 C outside temperature (perhaps
supersaturated solution with lack of crystallization cores?). I placed it into freezer to - 18C and checked the next day and there was white solid
product already. At this moment I had to go to Christmas and New Year holiday so I let it further in the freezer for 2 weeks (this is very likely
unnecessary but I had to go).
Then filtered on sintered funnel outside of building at - 6 C outside temperature and washed 2 times with 10 ml of ethanol (bottle of ethanol chilled
in freezer to -18 C) - both times after sucking out previous liquor and adding fresh ethanol the product was thoroughly stirred on the sintered funnel
with the fresh solvent using a glass rod and only then sucked.
Air dried inside at 20 C for 1 day, last 6 hours there was no weight loss (no evaporation of remainders of solvent anymore).
Yield 9,4 g (31,5 mmol, M=298,4 g/mol) = 94,5%
m.p. 100-102 C
This way does not require recrystallization and the yield is almost twice of the yield using 25% water ammonia solution which required 2
recrystallization which caused significant loses.

After 1 day at 0 C nothing crystallized at 0 C but after another day in freezer at -18 C there was a white product

dried product

[Edited on 12-1-2021 by Fery]
|
|
|
Bedlasky
International Hazard
    
Posts: 1243
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Very nice yield, Fery! Using ethanolic NH3 is good improvement  . .
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Hi Bedlasky, exactly. And also some disadvantages like scaring possible passersby on the street and neighbours... I did the NH3 absorption in ethanol
outside in my improvised wood-shed from pallets during dark night winter time. Perhaps the ammonia in ethanol solution does not need to be so much
concentrated (67 mmols = 1,13 grams enough theoretically and I dissolved 7,8 grams which is more than 5-fold excess, but certainly good to shift
equilibrium towards the product). And I also wanted to push limits.
Maybe also the lack of H2O shifted equilibrium towards the product as the water is one of the products.
[Edited on 12-1-2021 by Fery]
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
I found a video, someone did further steps in synthesis hydrobenzamid->amarin->lophin
https://www.youtube.com/watch?v=DJdCWzwEfq8
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
"The ready hydrolysis of hydrobenzamide by acids into benzaldehyde and ammonia is a characteristic reaction, but its stepwise decomposition by."
https://pubs.acs.org/doi/pdf/10.1021/ja01338a042
i cant quite find the text on the link itself but it explained this bit is in the link from google
anyhow- if one can react impure benzaldehyde with ammonia- maybe a more stable ammonia salt?- then, hydrolysis using acid would rapidly turn the
formed ammonia into a salt, thus one could use this as a possibly very neat purification method
|
|
|