roXefeller
Hazard to Others
  
Posts: 463
Registered: 9-9-2013
Location: 13 Colonies
Member Is Offline
Mood: 220 221 whatever it takes
|
|
Reduction mechanism
I've been searching and reading various sites. Admittedly I don’t have a good chemistry book handy. I've been looking at an aliphatic nitro
reduction using the zinc acetic acid process and found this image from this page. Can someone explain what is occurring? What is the SET acronym? Are the zinc and acetic acid reactants or catalysts? I read on the
smwiki about the AcOH reaction with zinc to release H2. Is that part of this reaction?
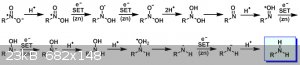
[Edited on 25-12-2020 by roXefeller]
One must forego the self to attain total spiritual creaminess and avoid the chewy chunks of degradation.
|
|
|
DBX Labs
Hazard to Self
 
Posts: 57
Registered: 24-12-2020
Member Is Offline
|
|
I am of the understanding that while acetic acid is consumed over the course of the nitro reduction, its use is largely for catalytic purposes.
However, even this doesn't make a whole ton of sense, given that in some instances, the reduction takes place in an environment depleted of free acid.
Granted, in place of acetic acid, an acetate salt is used instead.
https://patents.google.com/patent/US1990511A/en
Such is an example that I've spent much of the last month investigating to little avail.
Zinc is largely consumed in the sort of reduction you speak of. The ending slurry generally composes of zinc oxide and hydroxide.
I would believe that given there is a large excess of zinc in comparison to the present amount of acetic acid in most zinc/acetic reductions, the acid
is consumed early on, yielding zinc acetate which is likely the target reagent formed in situ.
Not sure if this answers or raises more questions.
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
SET is single-electron transfer. Bulk metallic zinc (with an active surface maintained by the acetic acid) transfers single electrons in a stepwise
manner to the substrate which is reduced through a series of radical/anion intermediates.
|
|
|
roXefeller
Hazard to Others
  
Posts: 463
Registered: 9-9-2013
Location: 13 Colonies
Member Is Offline
Mood: 220 221 whatever it takes
|
|
Thanks for the reply. So as I understand it, the acetic acid is an H+ donor to start the first step, because there is a charge imbalance in the nitro
resonance. Once this H+ is added, the group has a net positive charge and requires the e- donation from Zn to restore the charge imbalance. But this
leaves an unpaired electron, which attracts another donation from Zn. At this point Zn has donated enough to form a Zn(II)Acetate salt, allowing an
H+ transfer from a second acetate molecule to follow the e- transfer. Here's another image that may help more.
I guess at that point, the electronegativity of the oxygen attracts the hydrogens of the group and withdraws as a more stable unit leaving the N=O
group. This gets attacked again for its lone pair with available H+ from the available acid, causing further e- transfers to satisfy charge
imbalances and unfilled electron pairs. Zinc anions then pair with the acetate radicals.
If that all sounds right, can you answer this question. Why in this picture does there need to be strong acid to protonate the OH and not the
nitrogen?
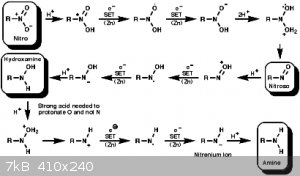
One must forego the self to attain total spiritual creaminess and avoid the chewy chunks of degradation.
|
|
|
roXefeller
Hazard to Others
  
Posts: 463
Registered: 9-9-2013
Location: 13 Colonies
Member Is Offline
Mood: 220 221 whatever it takes
|
|
I started considering what effect copper sulfate would have as a catalyst in this reduction. When I was reading websites discussing the use of copper
sulfate creating a galvanic cell with zinc, increasing the H2 production in sulfuric acid, most were indicating that it goes one way because of cell
potential. But the same copper sulfate is supposed to act as a catalyst in this reduction mechanism in concentrations of 0.2%. So I was trying to
think of how this could be.
I've come up with this effect, where the reduction of the R-NO2 group is more one-way than the reduction of copper. And by assuming that most of the
species are in equilibrium reactions except for the R-NO2 reaction, the equilibrium constants force the reaction to proceed, and moves species back
and forth while moving NH4 permanently to NH3 during the step-wise reduction, regenerating the Cu+2 but allowing it to still act as a galvanic cell.
I can make the image more descriptive if this sounds feasible.

One must forego the self to attain total spiritual creaminess and avoid the chewy chunks of degradation.
|
|
|