| Pages:
1
2
3
4
..
8 |
DraconicAcid
International Hazard
    
Posts: 4356
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by valeg96  | Oh, well, it makes sense then. I'm just not used to call CuCO3 or Cu(OH)2 a "metal base", and I believe I've never heard anyone call transition metal
hydroxides or carbonates "bases".
You're called DraconicAcid but you're caustic as hell today, bruh. |
Sorry- I've been spending the week trying very hard not to say what I really want to say to some of my students, and it's leaking out.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
valeg96
Hazard to Others
  
Posts: 254
Registered: 6-4-2014
Location: Italy
Member Is Offline
Mood: Moodless
|
|
No worries. I was double asking because if the "base" route is the only one, I'm not going to attempt turning my salts into bases. If it was some
kinda of "add salt, compound precipitates" thing I would've tried, but I don't want to fiddle around with them too much.
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 200
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
Quote: Originally posted by valeg96  | Oh, well, it makes sense then. I'm just not used to call CuCO3 or Cu(OH)2 a "metal base", and I believe I've never heard anyone call transition metal
hydroxides or carbonates "bases".
|
Sorry for being unclear, i said metal base because i used a variety of different basic forms of the metal ions, like CO3, HCO3, OH
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 200
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
Today i produced some more salts.
Sadly had to borrow the calcium one from Diachrynic because my calcium source i attempted it with was impure.
Very nice to see such good variety in the 2 group

I also produced some new amine ones, the color of the benzylamine salt is quite close to the Sr interestingly enough.
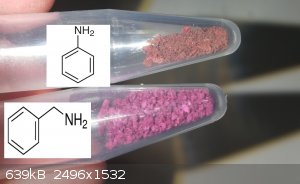
I also attempted to produce a urea salt, aswell as previously attempted a biuret salt. Neither of these worked, the violuric just crashes out alone,
but in solution they are both a dark purple.
[Edited on 17-12-2020 by RustyShackleford]

[Edited on 17-12-2020 by RustyShackleford]
|
|
|
valeg96
Hazard to Others
  
Posts: 254
Registered: 6-4-2014
Location: Italy
Member Is Offline
Mood: Moodless
|
|
What about Co, Ni, W, Cd, Hg, Pb? I'd like to try them myself but I don't want barbituric acid in my lab lmao
Also, I'm wondering what these compounds look like structurally. They can't be all just simple ionic salts, some are probably coordination compounds.
Also, are these compounds perfectly soluble, or do they crash out at some point?
[Edited on 17-12-2020 by valeg96]
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 200
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
Quote: Originally posted by valeg96  | What about Co, Ni, W, Cd, Hg, Pb? I'd like to try them myself but I don't want barbituric acid in my lab lmao
Also, I'm wondering what these compounds look like structurally. They can't be all just simple ionic salts, some are probably coordination compounds.
Also, are these compounds perfectly soluble, or do they crash out at some point? |
Co, Ni and Pb will definitely be made. i dont have any Cd though and not really comfortable making the Hg one. If you have either of those and would
like some violuric to try i could send you a couple grams if you cover the shipping and promise to post pics in this thread.
Some of the salts are really soluble (like the iron) and some are quite non soluble, like barium. As for the coordination and structure, the big
variety in colors likely stems from the resonance structures and that it has many ways of coordinating to metals. This is covered in the illumina
article and im sure you could find a lot of old research if you know how to search for that stuff.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Barbituric acid is very friendly toxicity wise, the toxicity is so benign the LD50 for rats is usually given to be > 5 gr/kg (read: couldn't be
determined).
It is the derivatives you have to be careful with.
|
|
|
valeg96
Hazard to Others
  
Posts: 254
Registered: 6-4-2014
Location: Italy
Member Is Offline
Mood: Moodless
|
|
Toxicity does not concern me. I'm not sure if it's controlled, over here.
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 200
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
Quote: Originally posted by Tsjerk  |
Barbituric acid is very friendly toxicity wise, the toxicity is so benign the LD50 for rats is usually given to be > 5 gr/kg (read: couldn't be
determined).
It is the derivatives you have to be careful with. |
Toxicity is very benign, and i dont think youd get in trouble over it (unless there are really stupid laws) because its not really feasible to produce
barbiturate drugs with barbituric acid.
[Edited on 17-12-2020 by RustyShackleford]
|
|
|
valeg96
Hazard to Others
  
Posts: 254
Registered: 6-4-2014
Location: Italy
Member Is Offline
Mood: Moodless
|
|
Quote: Originally posted by Tsjerk  | ]
Barbituric acid is very friendly toxicity wise, the toxicity is so benign the LD50 for rats is usually given to be > 5 gr/kg (read: couldn't be
determined).
It is the derivatives you have to be careful with. |
Read: "barbituric acid causes death by indigestion" 
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Here are some photos of violurate salt crystals.
 Barium violurate note the two different forms Barium violurate note the two different forms
 Strontium violurate, again note the two forms Strontium violurate, again note the two forms
 Calcium violurate, brick red spherules and bow-ties but sometime orange rhombs form, the yellow blades are UO2++ violurate, the greenish colour cause
by Fe2+ contamination of the mineral. Calcium violurate, brick red spherules and bow-ties but sometime orange rhombs form, the yellow blades are UO2++ violurate, the greenish colour cause
by Fe2+ contamination of the mineral.
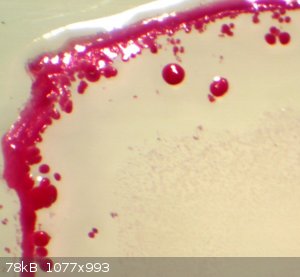 Sodium violurate forming at the edge of an evaporating drop Sodium violurate forming at the edge of an evaporating drop
Its worth noting that it seems rather difficult to grow only one specific form so Rusty's compounds may not be pure hence the colours may vary. I am
not sure what the difference is between the spherulitic or coralloid form and the euhedral crystals is. But I do remember from the Hantzsch paper that
some form "acid" and normal salts while other sometimes form hydrates. I will try and find the Hantzsch paper that I translated years ago.
[Edited on 17-12-2020 by Boffis]
[Edited on 17-12-2020 by Boffis]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4356
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I was starting to think that this could be a really cool experiment to add to the curriculum for my organic class, but since making the barbituic acid
to start with requires a 7 hour reflux, it probably won't happen this year. But I should be able to buy some over the summer.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Quote: Originally posted by woelen  | | [...]I do have diethyl malonate. Up to now I did not have any real use for it, I obtained it as part of a package of old chemicals, years ago, and it
still is in its unopened bottle. Now I have a use for that |
After studying the synthesis of barbituric acid, I unfortunately come to another conclusion. I have found info in one of my books and also online
sources. For making 100 grams of barbituric acid you need appr. 25 grams of sodium, 1 liter of absolute ethanol, 150 ml of diethylmalonate and
refluxing for appr. one working day (7 hours, 8 hours, that kind of times). This is a few tens of euros of chemicals and a full day of babysitting a
refluxing mix. I think that I also should buy it (appr. EUR 25 per 100 grams). Something for the beginning of next year.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4356
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
If you have nothing else to do with diethyl malonate, you could try making acac-like complexes with it.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2799
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Amazing work, had no idea this anion even existed, or that it was so colorful.
Quote: Originally posted by woelen  | | I do not know any other anion, which gives such striking different colors when e.g. Na(+) is exchanged with K(+). |
indeed, it seems Li+ would be a particularly interesting continuation
[Edited on 19-12-2020 by clearly_not_atara]
|
|
|
MidLifeChemist
Hazard to Others
  
Posts: 192
Registered: 4-7-2019
Location: West Coast USA
Member Is Offline
Mood: precipitatory
|
|
>> Amazing work, had no idea this anion even existed, or that it was so colorful.
That is what I wanted to say.
This is amazing work. I had no idea this anion existed, or that it was so colorful. I'm sure I speak for others too.
IMHO this is a great example of what makes chemistry so interesting. There is so much for all of us to learn. Many of us are doing things that
99.9999938% of the world has never seen or done.
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 200
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
Quote: Originally posted by clearly_not_atara  | Amazing work, had no idea this anion even existed, or that it was so colorful.
Quote: Originally posted by woelen  | | I do not know any other anion, which gives such striking different colors when e.g. Na(+) is exchanged with K(+). |
indeed, it seems Li+ would be a particularly interesting continuation
|
Thank you! I found out about this acid from Diachrynic so some credit to him for inspiring me to do this.
Lithium will likely be made by end of next weekend, as i have mailed some violuric to a friend to make it with. He will probably attempt rubidium also
and post results here.
Nickel is in the works by another user.
|
|
|
Justin Blaise
Hazard to Self
 
Posts: 82
Registered: 5-10-2011
Location: Parts Unknown
Member Is Offline
Mood: No Mood
|
|
Really cool that these are nicely colored and easily isolated. Do you have any Ce, Nd, or Bi salts to try to make some complexes?
|
|
|
RustyShackleford
Hazard to Others
  
Posts: 200
Registered: 10-12-2020
Location: Northern Europe
Member Is Offline
|
|
Quote: Originally posted by Justin Blaise  | | Really cool that these are nicely colored and easily isolated. Do you have any Ce, Nd, or Bi salts to try to make some complexes?
|
Sadly none of those
|
|
|
DraconicAcid
International Hazard
    
Posts: 4356
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Is the en salt with enH+ or enH2(2+)?
I imagine choline might also be interesting.
[Edited on 21-12-2020 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Advice needed please. My source did not have barbituric acid but he did have diethyl barbituric acid. So I got some to try. I dissolved 5g diethyl
barbituric acid in 100g hot water (with difficulty), and 3.5g sodium nitrite in 12g water. I added the sodium nitrite solution to the diethyl
barbituric acid solution......and nothing. No purple color change; the solution stayed clear. I left it stirring.
I have zero knowledge of organic chemistry so help will be appreciated. The obvious question being can diethyl barbituric acid be a starting point to
arrive at sodium violurate?
|
|
|
artemov
Hazard to Others
  
Posts: 181
Registered: 22-8-2018
Member Is Offline
|
|
Quote: Originally posted by Lion850  | Advice needed please. My source did not have barbituric acid but he did have diethyl barbituric acid. So I got some to try. I dissolved 5g diethyl
barbituric acid in 100g hot water (with difficulty), and 3.5g sodium nitrite in 12g water. I added the sodium nitrite solution to the diethyl
barbituric acid solution......and nothing. No purple color change; the solution stayed clear. I left it stirring.
I have zero knowledge of organic chemistry so help will be appreciated. The obvious question being can diethyl barbituric acid be a starting point to
arrive at sodium violurate? |
I probably can't help you, but are you talking about 1,3-diethylbarbituric acid or 5,5-diethylbarbituric acid  ? ?
If it's the latter, I dun think the oxime will form at the 5th position cos the acidic alpha hydrogen is gone? My very limited knowledge ....
If it's the former, maybe 1,3-diethylvioluric acid will form? Just guessing.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Hi Artemov it only says Standard Diaethylbarbitursaure / Diethyl-barbituric acid and a formula (C2H5)2CCONHCONHCO. A Swiss product made by Fluka AG
Buchs SG.
|
|
|
artemov
Hazard to Others
  
Posts: 181
Registered: 22-8-2018
Member Is Offline
|
|
Quote: Originally posted by Lion850  | Hi Artemov it only says Standard Diaethylbarbitursaure / Diethyl-barbituric acid and a formula (C2H5)2CCONHCONHCO. A Swiss product made by Fluka AG
Buchs SG.
|
Based on the formula, it seems that this is 5,5-diethylbarbituric acid aka barbital https://en.wikipedia.org/wiki/Barbital
This means the oxime cannot be formed at the 5th position to give violuric acid, unfortunately.
Do be careful with it cos it's a powerful sedative, and a controlled drug?
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Artemov thanks for the feedback. It will be returned to the source, no use to me then.
|
|
|
| Pages:
1
2
3
4
..
8 |