| Pages:
1
2
3
4 |
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
If you started with 5-Methoxy-indole, you might be able to proceed by the method of Somei, Yamada, and Tamura. They produced
4-Benzyloxy-indole-3-carbaldehyde in good yield, from Indole-3-carbaldehyde. First Psilocin synthesis I ever saw, that actually seemed reasonably
practical.
http://www.ch.ic.ac.uk/ectoc/echet98/pub/039/index.htm
|
|
|
Dextrose
Harmless

Posts: 34
Registered: 14-10-2006
Location: Denmark
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Sandmeyer
How many times are you gonna attach the picture of same route?  Imo, to make
4-subst indoles the best is to go via nitrene insertion as key step. i.e alkyl azidoacetate + aldehyde, cyclisation/nitrene insertion of the
condensation product, hydrolysis, decarboxylation... Imo, to make
4-subst indoles the best is to go via nitrene insertion as key step. i.e alkyl azidoacetate + aldehyde, cyclisation/nitrene insertion of the
condensation product, hydrolysis, decarboxylation... |
Hehe... I've been suggested so many other routes for indol-formation, but not this one though...
Can you please put up some graphics for me, please?
F.ex- the Heck-reaction - I have studied a bunch of publications with it - but i still can't figure out the reaction-mechanism directly to an indole -
how the heck ( ) does this one go? ) does this one go?
But it seems like 2-hydroxy-3-methoxy-6-nitrobenzaldehyde is commercial available, so it would be nice to find a cheap source for this item.
About the 4-hydroxylation's of the indoles... is that for fucking real? i've allways thought this to be next to impossible..!?
I just rememered that i have bunch of pure USP grade Melatonin, so that could be interesting...
[Edited on 16-10-2008 by Dextrose]
|
|
|
unome
Hazard to Others
  
Posts: 134
Registered: 17-10-2009
Member Is Offline
Mood: No Mood
|
|
There we go, on top of that Rhodium's pdf's include a procedure for a Hoffman degradation on an m-nitrobenzamide to the m-nitroaniline and I can think
of at least one japanese paper where they found that using H3PO4 to affect cyclization of the diamine to the 4-aminoindole gave the 4-hydroxyindole,
whereas sulfuric acid gave the 4-aminoindole.
In any event - using the 5-nitrobenzamide, you'd be able to reduce the nitro - form the phenylhydrazone with pyruvic acid - decarboxylate that, then
form the amide, etc. Hoffman to the amine - hot H3PO4 to remove it.
Alternatively, try chain lengthening that side (ie. use salicaldehyde instead of salicylic acid - extend the chain on that side to form the
phenylethylchloride and cyclize that to the benzofuran ie. an indole-type dragonFLY) or maybe put an MD on it - 4,5MDDMT should be fun
Attachment: Synthesis.4.nitroindole.ethylpyruvate.m.nitrophenylhydrazone.pdf (258kB)
This file has been downloaded 1729 times
Attachment: MW.nitration.benzoic.acid.m.nitrobenzoic.acid.pdf (45kB)
This file has been downloaded 816 times
Attachment: Synthesis.IndolecarboxylicAcids..pdf (192kB)
This file has been downloaded 867 times
Attachment: mw.nitration.salicylic.acid.pdf (63kB)
This file has been downloaded 1981 times
[EDIT - in any event, m-nitroaniline is as cheap and unwatched as anything you are likely to look at.
Also look at the last paper - where the author's use the phenylhydrazone of succinaldehydic acid (the Strecker degradation product of Glutamic acid)
and cyclize it with Sulfuric acid to get indole-3-acetic acid in pretty shit yields admittedly, but then again, it is all pretty cheap.
[Edited on 20-10-2009 by unome]
Attachment: Synthesis.Indoleacetic.Acids.Glutamine.pdf (279kB)
This file has been downloaded 1237 times
|
|
|
LabRatNW
Harmless

Posts: 18
Registered: 9-8-2009
Member Is Offline
Mood: No Mood
|
|
Adding things to the benzene ring of indole is horrifically difficult. You need build your benzene, close the indole, alkylate.
|
|
|
Methyl.Magic
Hazard to Others
  
Posts: 139
Registered: 14-5-2007
Member Is Offline
Mood: No Mood
|
|
Here is my pathway. Perhaps the reaction can be done on 5-MeO-DMT :
5MeODMT-> protection -> BuLi -> O2/red-> deprotection 

|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Holy necropost!
Sorry but I had to, since here is the most relevant thread discussing this topic, the preparation of psilomethoxin.
Based on the first three one-pot steps of the 4-benzyloxyl group introduction, I found a somewhat shorter possible route to accomplish the synthesis
of psilomethoxin, see the attached drawing.
Starting from 5-MeO indolecarboxaldehyde, the first reaction is clear, just as per the japanese paper.
But I found that the mannich reaction with eschenmosers salt is beneficial, as it introduces the N,N-dimethyl groups readily and is pretty high
yielding.
Of course the disadvantage is the beta ketone, but that can luckily under the right conditions be reduced to the alkane with plain NaBH4.
And since I realised the opportunity for this in the NaBH4 reduction, we can one-pot add some more borohydride followed by NiCl2, which is a reducing
system capable of O-debenzylation.
This makes four separate operations starting from 5-methoxyindole, when the second three and last two reactions are done one-pot.
I found that quite clever and wanted to share it on here too.
I know its not the most practical approach, but very feasible.
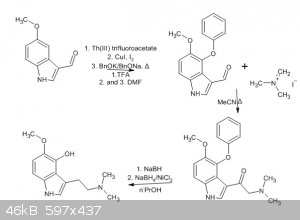
Edit: I just realised that I draw a 4-phenyloxy ether and not benzyloxy, I'm sorry, this is due to some properties of chemsketch....
I draw a 4-benzyl group and wanted to introduce the oxygen later(=replace one carbon with oxygen) and actually notice now for the first time that this
results in a carbon missing 
Please ignore that mistake and imagine a benzyloxy group instead, alright?
[Edited on 16-12-2020 by karlos³]
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Sorry, somehow over the course of time, the graphics have become kind of scrambled.
Psilomethoxine? Is that a thing?
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Yeah that trivial name turned up somewhere in the last few years and I have to say I think it is a really good one and sounds cool.
The graphics from Dextrose? But I can view them pretty clear?
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
One could also perform 4OH addition to the 5MeO DMT catalysed by our beloved fungus' enzymes  It would be the easiest way. It would be the easiest way.
[Edited on 16-12-2020 by mackolol]
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Its even easier and cheaper if you use plain 5-methoxytryptamine for that, the mushrooms also use N-methyltransferase enzymes and can dimethylate it
on their own.
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Although it could be toxic. Wiki states, that 4,5 dihydroxy Tryptamine is a neurotoxin (probably similar in action on serotonic receptors to the
action of 6 hydroxydopamine working on dopamine receptors, just killing them).
Would n,n - dimethyl protect our neurons from that? What do you think?
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Typed a long reply and lost it.
So the short answer is, no, if one of the hydroxyl is etherified, its not toxic.
Look next door I posted quite a few papers over the last half year.
Also, I heard anecdotal reports of people having doped mushroom substrate with 5-MeO DMT, and they report very distinct effects and a ca. 3x higher
potency, i.e. dosages even as little as a half gram of P. cubensis being already very strong.
And they also claim its enjoyable and nice.
If you want to go the biosynthesis route, well, use mexamine instead thats much cheaper and easier to make.
However I would recommend a synthetic route, even though its a lot of steps, that is a good exercise for one synthesis skills.
And the quantities to start with don't have to be overly huge anyways.
You would likely require to use the anthony-speeter though, but its just getting the reagents thats an issue with it, the reaction itself is very
simple and with Red-Al instead of LAH even very safe.
Or try the proposal of me above 
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
I'm pretty sure, I have seen it on Wikipedia and yes, I know it may not be the most reliable source, although 5MeO DMT is said to be metabolised
mainly by cyp2d6 which demethylates it to hydroxy derivative, I thought that's mainly the parent hydroxy compound that makes it work, similar way to
psilocybin -> psilocin although harder to hydrolyse.
I know that synthetic route is much more interesting and that's what we should discover, although I have a lot of different things going on in ma lab
that I want to finish and this is like a massive challenge. That;s why, I prefer to make it easier just for now and to be honest I would love to even
reach it to the 4OH substituted indole, which is challenging enough.
I'll look for your papers, thanks for advice, it's really easy to get the mexamine, so it may be really dope.
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
All right, now I see, the methoxy compound is methabolised in the gut and liver and the experiments about neurotoxicity were about the compound being
administered cranial. But wouldn't demethylated methabolites be absorbed in gut?
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Mexamine might be easier to obtain than Tryptamine.
I haven't cruised the net for things like that lately, but the last time I checked, Melatonin was pretty cheap in bulk. And, it is pretty easy to
hydrolyse.
Better check on that....
OK. I'm Back. Melatonin. $329.00 per Kilogram.
Yeah, I know in some parts of the world, guys can't buy it.
But, in the U.S., all you have to do is slap your money on the counter. The synthetic pathways into psychedelia are difficult enough, that it isn't
considered a problem.
https://www.bulksupplements.com/products/melatonin-powder?va...
OK. I checked the list. There are really a lot of places that guys can't buy Melatonin. But, it is still OK, in the U.S. and the UK?
I'll supply a link to that hydrolysis. Via a post from softbeard.
I have a hardcopy of the paper. But, softbeard has managed to to provide a live link to the actual journal article.
https://www.sciencemadness.org/whisper/viewthread.php?tid=79...
[Edited on 17-12-2020 by zed]
|
|
|
chemship1978
Harmless

Posts: 32
Registered: 8-6-2018
Member Is Offline
|
|
Quote: Originally posted by zed  | Mexamine might be easier to obtain than Tryptamine.
I haven't cruised the net for things like that lately, but the last time I checked, Melatonin was pretty cheap in bulk. And, it is pretty easy to
hydrolyse.
Better check on that....
OK. I'm Back. Melatonin. $329.00 per Kilogram.
Yeah, I know in some parts of the world, guys can't buy it.
|
https://www.aliexpress.com/item/4001352643390.html?spm=a2g0o...
55USD for 1kg here with free shipping to US. 
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
FYI, the listed prices on alibaba/aliexpress are more often than not either misleading or downright fake.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Aliexpress prices are usually fine, I would trust them as long as there is some positive feedback. Usually Aliexpress sellers do about anything to get
positive feedback.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
In europe, we lately got the option to purchase melatonin in food grade quality from S3.
Its pretty expensive but considered I paid much more for 1g(1,3g thanks to TCI) for 5-methoxyindole than for 10g of melatonin, then it is still pretty
neat to have that option for now.
Guess I'll go the mushroom route too experimentally, despite working on the synthetic method with a friend as well 
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Melatonin is one of those things I thought was OTC everywhere since you can get it so easily and cheaply in the states. Was it Rx only before?
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
It is still on prescription in my country.
But formerly we weren't able to buy it as a reagent except out of country.
Inside the EU it was hard to get.
And if you buy it, from example the US, it would go to customs and that means it can potentially be sent back as importat of medications is not
allowed for private persons.
I once ordered a nutritional supplement containing arachidonic acid, which I desired for certain experiments, and they hold it up and demanded so much
import tax(more than I paid!) that I decided to have it sent back instead.
If I knew these asses wouldn't refund me I would have paid that money, though.... arachidonic acid is almost impossible to get inside the EU, at least
in acceptable prices via nutritional supplement suppliers.
On the other hand, the expensive "herbal tea" bags I got from Peru once, only required me to pay import taxes and then I could take it back home.
Despite that the content was 1000x0,9g tea bags of mate de coca 
They haven't looked into it, just stopped it, and then paid taxes according to the shipping leaflet on the parcel.
But they have certain buzzwords to whom they act accordingly.
If anything comes as pills or capsule, they are especially interested in it.
So, melatonin powder in bulk might get through well.... I don't know of anyone who tried that.
It could be very different though since melatonin is mostly prescription only in europe.
At least in Germany it is, and if such a parcel would be checked by customs, you would be fucked.
Either a huge fine or have it sent back... although I doubt you would get it even if you paid the huge fine, though...
So in short, melatonin is scarce in europe, too hard to get.
We want to make 5-MeO tryptamines as well.... thats why I went per anthony speeter and bought expensive 5-MeO indole instead 
I guess we need to intensify the intercontinental relations for matters like this and have a friendly american send us bulk melatonine for an EU-wide
sharing action 
We probably have to organise such an event really....
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ummm. In general, 4 substituted indoles have always been excessively expensive, and hard to synthesize.
5-methoxy-indoles....not so much. You should be able to make yourself a jar-full as feedstock.
Now, where I live, those little Psilocybin mushrooms, grow like weeds. They pop-up everywhere. DMT and 5-Meo-DMT, can be extracted from the local
Phallerius Grasses., and if I were so inclined, I could buy the most potent Cannabis on the planet, at my neighborhood Pot-shop.
Apparently, most of the world isn't like that.
Anyway. 5-Methoxy-Indoles are available through the Fisher Indole Synthesis. From Phenyl-Hydrazones or Diazonium salts, of P-Anisidine.
Either 5-Methoxy-Tryptophol, or 5-Methoxy-Indole-Acetic Acid might be worthy targets.
Perhaps not ideal. But, synthetic pathways to Indoles, are limited, reagents are expensive or inaccessible, and...you have to start somewhere.
I know of the Speeter-Anthony, and I have heard it is reliable.
But, the required materials are fairly inaccessible here. Notably, Oxalyl Chloride. Expensive, hard to acquire, and very hard to make. The
requisite reagents tend to be highly restricted.
An obstacle that might be avoided, by utilizing the Indole Acetic Acid. Simply convert the acid to the Acetyl Chloride, in situ, and react it with a
suitable amine. This should yield an amide. Just utilize reaction conditions analogous to those in Tihkal's LSD procedure. This requires POCl3, but
that isn't impossible to make.
Were I in the mood to play chemistry right now. Indole Acetic Acid is cheap and available. Indole might not be. And, many of the other required....
Speeter and Anthony reagents, might as well be on the moon.
Yeah, OK. I could make a lot of it, but I really lack motivation.
What can I say? I've gotten old and lazy!
[Edited on 17-12-2020 by zed]
[Edited on 18-12-2020 by zed]
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Yeah well, I used the anthony-speeter, on 5-methoxy-, 5-bromo-, and plain indole, reducing the glyoxylamides with Red-Al(expensive, but easier to
use), that is a very viable method, even for the halotryptamines since it doesn't result in dehalogenation unlike LAH.
I've even been told(and have the ref somewhere) that diborane in-situ prepared is an alternative to using LAH, and that is definitely very accessible
too.
For completeness sake, I add the diborane reduction of indolglyoxylamides.
That makes oxalyl chloride the only remaining real hurdle for the anthony-speeter, but the advantages are that its really an idiot proof method.
My yields could be improved, but it worked free of any failure on my first trial.
And we here at the forum have the one or other guy at hand who can organise that, so this is really not an impossible task to get it.
Now, in this special case here, for psilomethoxin, we don't have as many options for the preparation of the starting material.
And we are pretty much restricted with the need to use the route via indole, almost.
The route I gave above(inspired from your ref zed) is one of the very few exceptions we have here.
Otherwise, with the need to start from the indole, what do we have left?
We can go via FC-acylation to the acetylindole, a-bromination, amination and finally reduce the ketone with borohydride... the downside is, some of
these steps aren't very high yielding.
And not to forget, at that point we have already done 3-5 reactions to prepare the starting indole... so we can't afford to waste some on going a
moderately yielding route.
The fischer indole is out of question too, since 3-hydroxy(or 3-acetoxy)-4-methoxy phenylhydrazine is going to yield with around a 4:1 preference the
unwanted 5-MeO-6-HO indole.
No matter if you go to the tryptophol via the fischer or directly to the tryptamine, so thats not a viable choice.
So what else is left, gramine, going via the nitrile for example, maybe the 3-indolyl grignard reacted with that toxic blister agent, the mustard gas
analogue, or directly with ethylene oxide for tryptophol maybe?
And surely the one or other approach I don't have in mind right now.
Mostly some greatly skill-dependant ways for the chemists.
That means the only really realistic approach, if you want to make psilomethoxin, remains the anthony-speeter.
The only potentially low yielding step at this point is the final reduction in the worst case, making that the most preferred approach.
The Marc Julia et. al. paper that even Shulgin referred to, the first full synthesis of psilomethoxin, used that method too.
And we can't really introduce the 4-hydroxy group afterwards, we definitely have almost no choice besides having to construct the indole ourselves
first.
Note, this 4-hydroxy-5-methoxyindole has just as few, even fewer, options for its synthesis than plain 4-substituted indoles.
Its not easy to make, definitely another league than the simple unsubstituted or 5-substituted tryptamines who are comparably simple to make.
This is a real challenge and that is why Shulgin never made it apparently.
Even though he made the 4,5-MDO tryptamines which aren't really harder to prepare, I would say its even around the same level of difficulty, just
requires one or two reactions in addition... and one or two in addition to make the benzaldehyde precursor.
But manageable.
I am attempting this with a friend and we are starting from ortho-vanillin via the "classical" route.
Tosylation of the o-vanillin, followed by nitration, followed by exchange of the tosylate with an acetyl group, followed by nitrostyrene formation and
reductive cyclisation with Fe/acid reduction.
I've saved a small rest of oxalyl chloride and still have enough red-al for a few grams of the glyoxylamide left for this.
If I would have to do the first reactions, I would have started from 5-methoxy-2-nitrophenol with a VH-formylation, to skip the nitration step which I
would prefer not needing to do it.
And then essentially the same, except that there is no need to go via tosylation(which is just done because the sulfonylate ester of o-vanillin
nitrates selectively on the right position), we can directly acetylate the phenolic hydroxy, and after those two steps its just nitrostyrene formation
and reduction to give 4-acetoxy-5-methoxyindole.
4-HO indoles are really damn hard to make, but this 4-HO-5-MeO indole(or its 4-esterified analogue) is really the icing on the cake in terms of effort
thats required to make it, and sadly there are not many alternative routes existing.
Maybe one of those indole syntheses who make use of anilines or such, but else the options are pretty limited here.
Thats why I was pretty proud on my proposal above, via 5-MeO 3-indolcarboxaldehyde, its probably the shortest imaginable route to psilomethoxin with
my improvements.
Although of course with the problematic downsides, of the need to introduce the 4-benzyloxy group via thallation with a toxic thallium salt, which
needs to be substituted with expensive iodine, before the benzyl ether is finally attached.
Yeah, the dimethyl(methylene)ammonium chloride/iodide has to be made in at least two separate steps, or has to be bought for a way too expensive
price.
And lastly, 5-MeO indole has to be bought and thats neither nonsuspicious nor very accessible, and neither cheap.
But besides that, and the not so nice yield in the final reduction to the tryptamine(but then again, good yield in the O-debenzylation with
NaBH4/NiCl2), it really does not have much disadvantages.
Its uneconomical because of all the separate pre-precursor preparations though.
So, the best approach is still the classical variation, via the leimgruber-batcho variation(Nenescu? what was the rumanian chemists name? has his own
indole synthesis, a different one, who invented the reductive ortho-nitro nitrostyrene cyclisation variant of the L-B indole synth).
And from the indole straight forward to the glyoxylamide and its reduction.
Thats good and solid manual crafting, not short and clever but really practical work.
Attachment: biswas1968.pdf (1.3MB)
This file has been downloaded 449 times
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Mmmm. Buy enough of it, and the price of Oxalyl Chloride is decent.
Is Sigma friendly in Europe? I no longer have relationships for exotic chemicals in the U.S..
Precurser PCl5 which is restricted by International Chemical Weapons Treaties, is a hard get.
Likewise, Phosphorus itself, is a tough get, in the U.S., and making everything from scratch is arduous.
Perhaps Oxalyl Chloride is cheaper, and more readily available, than its alternatives.
https://www.sigmaaldrich.com/catalog/product/ALDRICH/221015?...
Very reasonable price if you buy 10 Kilos. I don't know if any carrier will fly it, knowingly.
That the desired intermediate can be synthesized via the 5-Methoxy-Indole-3-Aldehyde, is not certain. Unless of course, someone has successfully done
it, and provided details.
Were I to embark on attempting that synthesis, I would make about a hundred grams of 5-Methoxy-Indole to supply my experiments. It is purported that
P-Anisidine Phenylhydrazone of Ethyl Pyruvate, will produce the Indole-2-carboxylic acid ester in decent yield. The 2-carboxylic acid is then be
decarboxylated.
Might be a. better synthesis out there. I don't know.
It's been a while. There is often a better procedure somewhere.
I just know, I'd want a healthy supply of the critical intermediates.
[Edited on 18-12-2020 by zed]
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Quote: Originally posted by zed  |
That the desired intermediate can be synthesized via the 5-Methoxy-Indole-3-Aldehyde, is not certain. Unless of course, someone has successfully done
it, and provided details. |
Yes, it is of course just an assumption.
We know enough about aromatic thallation on the other hand.
5-MeO is activating.
It seems that for the thallation of anisole a preferance of ca. 80% para opposed to 20% ortho is expected....
I think this can't be that simple compared to the higher substituted indolylcarbaldehyde in our case, but if, then this means mostly the undesired
7-substituted analogue and in turn my idea to be of no value.
However, I've seen metallations of 3-substituted 5-haloindoles that would add on position 4-, of course those are mildly deactivating, I just mention
this because from a synthetic position the 4-HO-5-x disubstitution is almost as useful.
But after thinking some more, I fear you are right.
The 5-MeO group meddles with the regioselectivity of the thallation and would give 5-MeO-7-HO probably.... damn, that sucks.
It still remains a nice approach to 4-HO tryptamines, and is thus very valuable.
The remainder of my proposed route, i.e. mannich with eschenmosers salt in special, followed by the reduction to the tryptamine remains still a
valuable and very short variation, most straight forward.
And that reminds me of this, something surprising and unexpected: a friend had analysed mushrooms grown on 5-MeO DMT spiked substrate.
And they haven't contained the desired tryptamine, instead just the usual stuff and another "unknown" compound.
So this indicates that the 5-MeO substitution interfers with the 4-hydroxylase enzyme.
There is actually no short biosynthetic preparation possible for this analogue, which even Shulgin had thought to work.
This substance can only be accessed via synthetic means.
Quote: Originally posted by zed  |
Were I to embark on attempting that synthesis, I would make about a hundred grams of 5-Methoxy-Indole to supply my experiments. It is purported that
P-Anisidine Phenylhydrazone of Ethyl Pyruvate, will produce the Indole-2-carboxylic acid ester in decent yield. The 2-carboxylic acid is then be
decarboxylated.
Might be a. better synthesis out there. I don't know.
It's been a while. There is often a better procedure somewhere. |
Interesting, I would have never expected that the 2-carboxylic acid can that easily be removed, of course I knew about that fischer variation but
always thought that would require more effort.
That makes the preparation of substituted indoles more accessible, good to know!
Yes, logical.
Since we've already decided to use the standard route, proven to work, used with success and affordable, we start with 100g ortho-vanillin.
That sound like a sufficient quantity, even half of that would probably be enough to obtain a few grams, even with intentionally lowered yield
expectations.
I already wonder how much of the disubstituted indole will be available when its time to do the speeter-anthony.
But then again, the last three steps are a walk in the park, also I've started with merely above a gram of a substituted indole and still got a very
nice quantity of the final tryptamine out.
So this should be the easiest part of the whole synthesis.
|
|
|
| Pages:
1
2
3
4 |
|