EverythingAl2O3
Hazard to Self
 
Posts: 51
Registered: 3-9-2019
Member Is Offline
|
|
Questions about MPV reduction
I found this paper on an alternative catalyst for MPV reduction instead of using AliPr3 and had a few questions about this paper and MPV reductions in
general.
In the paper it lists the general procedure as 1 (1.0 equiv,
0.10 mmol), K3PO4 (0.50−4.0 equiv), reductant (2.5 equiv), and 1,4-
dioxane (1.0 M) heated at 80−120 °C for 16 h in a sealed vial under
an atmosphere of N2.
1. Is the 1,4-Dioxane (1.0 M) 1 mole of dioxane or is it 1 molar dioxane? If it is 1 molar dioxane what the heck does that mean for the amount you
need to add?
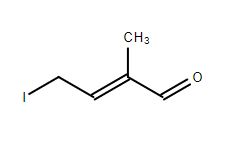
2. Is the MPV reduction tolerant of halogen groups? I want to reduce 4-iodo-2-methylbut-2-en-al (will post a picture) to 4-iodo-2-methylbut-2-en-1-ol
and want to make sure it won't get messed up. I don't trust other reductants like LiAlH4 or NaBH4 to not reduce the double bond or the iodine.
3. Since K3PO4 is a catalyst for this reaction and AliPr3 is also a catalyst, does anyone think AlPO4 could also have similar activity?
Attachment: MPV.pdf (925kB)
This file has been downloaded 406 times
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
It usually means dioxane to make a 1 M solution of substrate in the dioxane.
I would think the original mpv reduction may be more suitable here, especially since you've got that iodide in there but the only way to find out is
to try it.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2799
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
| Quote: | | Is the MPV reduction tolerant of halogen groups? |
http://en.wikipedia.org/wiki/Williamson_ether_synthesis
No 
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ummm. MPV. Might expect either reaction of that Iodide, or synthesis of an Ether.
I'm not sure about reduction of the double bond, but ordinarily not easily reduced by ordinary reagents.
Sodium Borohydride probably would leave the double bond alone, and reduce the aldehyde.
Action on that Allyl type Iodide? I don't know.
Anyway, if you don't have a reference, this could be touchy.
I'll check.
What I would call conjugated systems. The Iodine might be especially reactive.
The double bonded C=C.... Normally, not reduced by Hydrides, might be vulnerable in that configuration.
Ummm. Looks like you need a trip to the library. Find a reference for preparing your target molecule. Or, find another way.
[Edited on 9-12-2020 by zed]
[Edited on 9-12-2020 by zed]
|
|
|
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
I’m not sure about aldehydes but I do have a paper on reduction of esters with magnesium in methanol. It could possibly be used to reduce the double
bond without touching the aldehyde, not entirely sure. You could make the ester and then after double bond reduction use DIBAL-H to reduce it back to
an aldehyde, but it may not be the best of yields.
Here’s the paper
Attachment: Magnesium reduction of a,b-unsaturated esters using Mg in methanol (135kB)
This file has been downloaded 375 times
|
|
|
EverythingAl2O3
Hazard to Self
 
Posts: 51
Registered: 3-9-2019
Member Is Offline
|
|
| Quote: |
Ummm. MPV. Might expect either reaction of that Iodide, or synthesis of an Ether.
I'm not sure about reduction of the double bond, but ordinarily not easily reduced by ordinary reagents.
Sodium Borohydride probably would leave the double bond alone, and reduce the aldehyde.
Action on that Allyl type Iodide? I don't know.
Anyway, if you don't have a reference, this could be touchy.
I'll check.
What I would call conjugated systems. The Iodine might be especially reactive.
The double bonded C=C.... Normally, not reduced by Hydrides, might be vulnerable in that configuration.
Ummm. Looks like you need a trip to the library. Find a reference for preparing your target molecule. Or, find another way.
|
I have thought of a work around. I was too busy looking at the wrong thing. I think I can make an alcohol where the double bond was, removing the
conjugation, then do MPV, the aldehyde should react faster than the alcohol and then I will be left with a diol, react with conc. H2SO4 (or less if I
don't need conc.) and get the desired product in that manner. Then it will keep the desired form for the Sn2 reaction afterwards.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2799
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Opylation: Reduction with Mg will reduce the iodide.
EverythingAl2O3: First of all, it does not matter, you cannot do the MPV reduction on an allyl iodide, it produces an ether, as I
said already.
However, even if that were not a problem, you have the additional issue of stability of the alcohol: beta-hydroxyaldehydes tend to eliminate, while
geminal diols undergo pinacol rearrangement instead of elimination when treated with acid.
Instead, consider substituting prenol with hydriodic acid to prenyl iodide, and Riley oxidation with SeO2
|
|
|
EverythingAl2O3
Hazard to Self
 
Posts: 51
Registered: 3-9-2019
Member Is Offline
|
|
| Quote: |
EverythingAl2O3: First of all, it does not matter, you cannot do the MPV reduction on an allyl iodide, it produces an ether, as I said already.
However, even if that were not a problem, you have the additional issue of stability of the alcohol: beta-hydroxyaldehydes tend to eliminate, while
geminal diols undergo pinacol rearrangement instead of elimination when treated with acid.
Instead, consider substituting prenol with hydriodic acid to prenyl iodide, and Riley oxidation with SeO2
|
I understand that I can't MPV with allyl iodide. Which is why I'm no longer trying to do that. I also see your point with reversing the conjugatiin.
So if I stop at the aldol addition product and do not heat the reaction it should leave me with the aldehyde and secondary alcohol. Do MPV to reduce
the aldehyde to get a 1,3 diol. Then treat with strong acid to eliminate the secondary alcohol. Then I should have my product. Please correct me if I
misunderstood anything that you said or if I am creating even more trouble for myself with this work around (I'm trying so hard to stick with this
method because I already have most of the reagents to make it)
I looked up Riley oxidation and that is a very useful reaction. I'm gonna look into that for this synthesis, it might be easier. Do you have any
procedures on hand that would give the relative conditions? Thanks
|
|
|
EverythingAl2O3
Hazard to Self
 
Posts: 51
Registered: 3-9-2019
Member Is Offline
|
|
| Quote: |
I understand that I can't MPV with allyl iodide. Which is why I'm no longer trying to do that. I also see your point with reversing the conjugatiin.
So if I stop at the aldol addition product and do not heat the reaction it should leave me with the aldehyde and secondary alcohol. Do MPV to reduce
the aldehyde to get a 1,3 diol. Then treat with strong acid to eliminate the secondary alcohol. Then I should have my product. Please correct me if I
misunderstood anything that you said or if I am creating even more trouble for myself with this work around (I'm trying so hard to stick with this
method because I already have most of the reagents to make it)
|
I now understand why this is wrong. I was trying to keep the iodine on because I wanted to save it for Sn2 with Adenine. I see now that I can't really
make that work with MPV. So for anyone who sees this.
If I just went ahead and did Sn2 with adenine,I have an non-enolizable aldehyde, so I should just be able to do a cannizzaro reaction right? Unless
amine groups interfere with cannizzaro like MPV reduction.
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
It would appear, the reduction itself, via MPV, is quite doable. The question revolves around the reactivity of the Iodine. It can behave in ways
that are counter-intuitive.
2-Buten-l-ol: ' Crotonaldehyde (210 g, 3 moles) and dry 2-propanol (11) are added
to a solution of aluminum isopropoxide prepared in the usual way from aluminum (47 g, 1.74 moles) and 2-propanol (500 ml). The reaction is carried out
in a 2-1 round-bottomed flask fitted with a Vigreux column to which a descending condenser is attached. The mixture is heated in an oil-bath (bath
temperature 110°), the acetone formed distilling slowly at 60-70°. When no more acetone can be detected in the distillate by
2,4-dinitrophenylhydrazine (after 8-9 h), the residual 2-propanol is removed under diminished pressure. The residue is cooled to 40° and hydrolysed,
with cooling as necessary, by cold 6Nsulfuric acid (900ml; from 145 ml of concentrated acid and 790 ml of water). The organic phase is separated,
washed with water, and distilled at 60-70° at a pressure decreasing gradually from 275 to 65 mm. Finally, distillation is completed at 100°/20 mm. A
second fraction of 2-buten-l-ol is obtained by distillation of the aqueous phase and saturation of the distillate with potassium hydroxide. The crude
2-buten-l-ol is dried over potassium hydroxide and distilled through a column. It has b.p. 117-122° and the yield is 60% (130g).
From our library.... "Preparative Organic Chemistry"
If you should be so fortunate as to accomplish the reduction with Iodine present, clearly drying with KOH, is probably not a good idea.
https://en.wikipedia.org/wiki/Aluminium_isopropoxide
[Edited on 13-4-2021 by zed]
|
|
|
dawt
Hazard to Self
 
Posts: 74
Registered: 9-5-2016
Location: EU
Member Is Offline
Mood: fluorescent
|
|
Off-topic, but thanks for posting that paper. I've been looking for a simple way of making hydrocinnamic acid and this is very welcome news to me. The
electroreduction of cinnamic acid in Org. Syn. is kinda clunky, and I didn't want to deal with sodium and benzyl chloride (via malonic acid
esters) if it could be avoided. Just posting this so it turns up in the search results.
Kwon Youn, I., Hwan Yon, G., & Siek Pak, C. (1986). Magnesium-methanol as a simple convenient reducing agent for α,β-unsaturated esters.
Tetrahedron Letters, 27(21), 2409–2410. https://doi.org/10.1016/s0040-4039(00)84542-6
|
|
|