Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
Question about organic electroluminescent synthesis
Hello all, I’ve been looking into the production of electroluminescent displays and watched a couple of Ben Krasnow’s videos on screen printing
and such. I wanted to plan out a build for an OLED segment display and wanted to start from the ground up. I’ve already planned out how to make
‘PEDOT’:’PSS’ and a couple other required materials but for the actual organic light emitter I wanted something that was reasonably
synthesized. I found that pentaphenylcyclopentadiene is a blue light emitter and seems like a reasonable organic electroluminescent compound.
Now my plan to synthesize this was to prepare tetraphenylcyclopentadienone via a double aldol condensation of benzil and dibenzylketone, then react it
with a phenylmagnesium halide Grignard reagent to form 1-phenyl-1-hydroxytetraphenylcyclopentadiene. At this point I thought a simple dehydration
reaction with concentrated sulfuric or phosphoric acid would finish the job but seeing as such reaction proceed via a carbocation and there are no
available carbons to for a double bond I’m not so sure it will work. Would possibly adding formic acid into the mix as a hydride transfer reagent
work? Or am I overthinking this and a simple dehydration reaction do the job?
I’ll post a couple pictures about the compounds I’m referencing below. Let me know what you think.
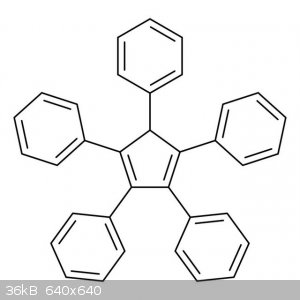 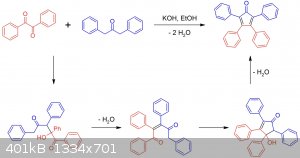 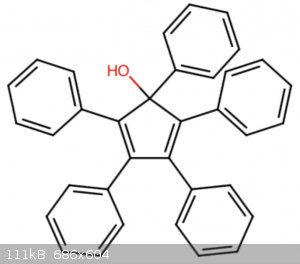
Edit: for anybody interested I have a paper about different organic electroluminescent compounds that lists pentaphenylcyclopentadiene as an emitter
of 465nm light at 120 lux
[Edited on 1-12-2020 by Opylation]
|
|
|
beta4
Hazard to Self
 
Posts: 56
Registered: 3-2-2019
Member Is Offline
|
|
Making OLED display (as well as inorganic EL displays) is one of my long-term projects, currently stuck due to lack of time.
While I can't help you with pentaphenylcyclopentadiene, I found this link where someone made working OLEDs which may contain useful information: https://hackaday.io/project/8012-homemade-oleds
My plan is to make Tris(bipyridine)ruthenium(II) chloride and just buy the PEDOT : PSS, however I am at a very early stage. I only made pyridine and
my next step will be to make the 2,2'-bipyridine.
|
|
|
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
I was trying to avoid using any rare earth metals, or at least the expensive ones. That’s for the head up though, I’ll have to check it out
sometime soon. Another simple emitter to synthesize is tetraphenyl butadiene. In fact I think it’s 5 times more luminous than
pentaphenylcyclopentadiene. As far as purchasing ‘PEDOT’:’PSS’ I try to avoid purchasing specialized chemicals if I can. The exorbitant prices
make the cheap part of me have a heart attack. Plus there’s no better feeling then building something from from scratch. I remember when I build my
first DC power supply out of transistors, capacitors, and diodes and I thought that thing was so cool. Needless to say it wasn’t the latest or
greatest but I made it
[Edited on 1-12-2020 by Opylation]
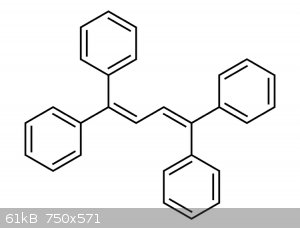
|
|
|
beta4
Hazard to Self
 
Posts: 56
Registered: 3-2-2019
Member Is Offline
|
|
I agree about the issue of expensive reagents, that's why I haven't bought ruthenium chloride yet.
I'm waiting to see if I can make the bipyridine first.
However, sometimes when you factor in the cost of all the reagents needed to make a specific compound, plus the poor yield of some steps as well as
the failed reactions, sometimes it's cheaper to just buy some reagents. And since I found PEDOT : PSS on ebay I'll likely just buy it.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2753
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
from https://link.springer.com/article/10.1007%2FBF00698457 (I can't download the article, but the abstract says it all,
"An improved method for the synthesis of pentaphenylcyclopentadiene is suggested. It involves the reaction of tetraphenylcyclopentadienone with PhMgBr
and subsequent treatment of the reaction mixture with an excess of LiAlH4 in THF. The effect of arylating agents and reducing agents on the yield of
the target product is studied. The method suggested can be used for synthesizing other polyaromatic cyclopentadienes, in particular,
1,2,5-triphenyl-3, 4-(1,8-naphthylene)cyclopentadiene."
So LAH is used to remove the hydoxyl group. Might work other ways as well. The benzoin can be made from benzaldehyde and the dibenzyl ketone we
made some way also, but can't remember, I'm sure it was not too bad.
I made tetraphenylcyclopentadienone in my organic lab a few decades ago, and that prep works. The tetraphenylnutadiene looks easy to make solely
from the symmetry, likely can be made from some benzophenone chemistry or from diphenylmethylchloride. Maybe a Wittig or some other related way.
|
|
|
Opylation
Hazard to Others
  
Posts: 131
Registered: 30-8-2019
Member Is Offline
|
|
Okay that is good to know. They use reducing agents to remove the hydroxyl group. I have NaBH4, actually quite a bit of it since its cheaper to get it
overseas in bulk, so this is not out of my reach of production. I know NaBH4 is not as strong as LiAlH4 but it's worth a try. Thanks for the link to
the article, I will have to take a look to see if i can get some more information out of it.
EDIT: ouch! I took a look at that article and they listed a 0% yield using PhMgBr and NaBH4 so look like that's out the window.
[Edited on 3-12-2020 by Opylation]
|
|
|
beta4
Hazard to Self
 
Posts: 56
Registered: 3-2-2019
Member Is Offline
|
|
Bought the PEDOT : PSS from ebay. Tried the bipyridine synthesis in January, but that failed and turned most of my pyridine to tar. Been processing
the tar since then to try and see if there's some product in there, but I get bored quickly and give up only to try again a few months later. Making
OLED materials is NOT easy.
[Edited on 23-7-2022 by beta4]
|
|
|