| Pages:
1
2 |
MidLifeChemist
Hazard to Others
  
Posts: 192
Registered: 4-7-2019
Location: West Coast USA
Member Is Offline
Mood: precipitatory
|
|
What are most dangerous toxic chemicals you are comfortable handling in your home lab?
I'm wondering where people draw the line regarding the most toxic chemicals they are comfortable handling in their home lab, and what, if any
additional precautions they take other than the normal PPE (goggles, gloves, lab coat).
I was able to recently set up a home lab in my garage now that I have a larger space. But I don't have a fume hood, and I have other family members in
the house. I thoroughly read the safety data sheet on all chemicals I have to understand the risks.
Here's the most toxic chemicals I have now:
- Top of the list out the chemicals I use is (imho) Potassium Dichromate because of risks from several routes - 130mg oral LD50
(rat, per kg), OSHA hazard ids are acute oral 3, acute inhalation (dust) 2, carcinogen 1A, organ toxicity 1
For hazard ids, lower number means higher risk. More info here: https://www.osha.gov/Publications/OSHA3844.pdf
Other top carcinogens I have & use include:
- Vanadium Pentoxide - 300mg LD50, acute oral 4, inhalation 4, carcinogen 2, organ 1
- Nickel sulfate - 264mg LD50, acute oral 4, carcinogen 1A
I also have CoSO4 and SbCl3 which are slightly less dangerous based on safety data sheets.
Topping my acute toxicity list of chemicals I use are:
- Sodium fluoride - 31mg to 100mg LD50, acute oral 3; this is the most acutely toxic chemical I have
- Lead nitrate - 93mg LD50, acute oral 4, inhalation 4, organ 2
- Barium chloride - 115mg LD50, acute oral 3, inhalation 4
- Sodium sulfide - 250mg LD50, acute oral 3, acute dermal 3, included because of the risk of producing highly toxic H2S with acid
I'm comfortable with what I have, but the following are chemicals I'd like to have & use but I'm holding off on because of their
toxicity and overall nastiness of symptoms, and my comfort level with them in my current space isn't quite there yet:
- Mercury II chloride - 26mg LD50, acute oral 2, acute dermal 1, organ toxicity 1
- Sodium arsenite - 41mg LD50, acute oral 2, dermal 2, inhalation 3, carcinogen 1A
- Thallium chloride - 35mg LD50, acute oral 2, inhalation 2, organ toxicity 2
- Cadmium nitrate - 100mg LD50, acute oral 3, dermal 4, inhalation 4, carcinogen 1A, specific organ 1
- Sodium selenate / selenite - 2mg to 7mg LD50, acute oral 1, acute inhalation 3
So what are the most toxic chemicals that other SM members have? Where do you draw the line with respect to what chemicals you will store and use in
your home lab? And do you take additional precautions with your most toxic chemicals?
Looking forward to reading the responses...
[Edited on 11/29/2020 by MidLifeChemist]
|
|
|
Fluorite
Hazard to Others
  
Posts: 139
Registered: 26-12-2018
Location: United Arab Emirates
Member Is Offline
|
|
the most toxic chemicals I have: sodium chromate, sulfur monochloride, and nitric acid
Why toxic chemicals are stable? Chromic acid is stable but carcinogenic af but permanganic acid and ferric acid are less toxic but unstable 
Sodium fluoride is very toxic? I thought it's safe to touch https://m.youtube.com/watch?v=scN6_LItWqo
I need to understand what acute oral, dermal, inhalation means :/
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Are the amounts per kg or total dosage? Because sodium fluoride lethal dose is 7-10 grams according to Wiki. This puts it in the range of caffeine,
hence you can handle it pretty casually with common sense. Brits got it on poisons list, though, so it should be more toxic than a cuppa, at least.
(pun for the nanny banny state)
V2O5 is also available in ceramics and artisan stores sold by the bag, so if it's actually almost as toxic as cyanide, I find there a big how?
I don't actually handle toxic stuff at all unless I have access to my exhaust connected fume hood. With that, I feel much safer handling even the more
toxic stuff, unless it has risk of blowing up, splashing or causing any sudden runaways or stuff like that. And yeah, sulfuric acid is toxic too, but
it's not volatile and is easily decontaminated, so you get the point on toxicity.
Toxicity has little to do with stability. It has more to do with our metabolism, which is just sensitive to some stuff. There are ubiquitous amounts
of molecules that are very unstable and at the same time basically nontoxic.
Some toxic stuff are volatile or dust a lot so they can be breathed. Smaller the molecule, the easier it passes through the skin, hence dermal
exposure can provide fatal, see cyanide solution. Oral toxicity is as invasive way of getting stuff into your body, but metabolism states if it's
absorbed. Metallic mercury and non-reactive, non-water-soluble stuff are generally sparsely absorbed. Cyanide salts hydrolyse with water already, and
in contact with stomach acid will make HCN, and the rest is history.
Sometimes it is stated that if something toxic is ingested, one should not be induced vomiting. I wonder if this is less harmful than puking it out as
fast as possible, meanwhile it is absorbed in the body causing systemic damage.
Fun fact, hydrofluoric acid does not state instructions in case it's ingested. It's unnecessary by that time.
|
|
|
HydrogenSulphate
Harmless

Posts: 38
Registered: 13-10-2019
Member Is Offline
Mood: Caffeinated
|
|
Quote: Originally posted by Fyndium  | Are the amounts per kg or total dosage? Because sodium fluoride lethal dose is 7-10 grams according to Wiki. This puts it in the range of caffeine,
hence you can handle it pretty casually with common sense. Brits got it on poisons list, though, so it should be more toxic than a cuppa, at least.
(pun for the nanny banny state)
V2O5 is also available in ceramics and artisan stores sold by the bag, so if it's actually almost as toxic as cyanide, I find there a big how?
I don't actually handle toxic stuff at all unless I have access to my exhaust connected fume hood. With that, I feel much safer handling even the more
toxic stuff, unless it has risk of blowing up, splashing or causing any sudden runaways or stuff like that. And yeah, sulfuric acid is toxic too, but
it's not volatile and is easily decontaminated, so you get the point on toxicity.
Toxicity has little to do with stability. It has more to do with our metabolism, which is just sensitive to some stuff. There are ubiquitous amounts
of molecules that are very unstable and at the same time basically nontoxic.
Some toxic stuff are volatile or dust a lot so they can be breathed. Smaller the molecule, the easier it passes through the skin, hence dermal
exposure can provide fatal, see cyanide solution. Oral toxicity is as invasive way of getting stuff into your body, but metabolism states if it's
absorbed. Metallic mercury and non-reactive, non-water-soluble stuff are generally sparsely absorbed. Cyanide salts hydrolyse with water already, and
in contact with stomach acid will make HCN, and the rest is history.
Sometimes it is stated that if something toxic is ingested, one should not be induced vomiting. I wonder if this is less harmful than puking it out as
fast as possible, meanwhile it is absorbed in the body causing systemic damage.
Fun fact, hydrofluoric acid does not state instructions in case it's ingested. It's unnecessary by that time. |
I believe that phenol is also on the poison list in 'Banny Nanny' state, which is probably why it is nigh on impossible for a private individual
residing in 'Banny Nanny' state to buy it (although podiatrists are able to procure it). I mean, yes, phenol can potentially be quite harmful (for
example, inflict burns via the denaturation of proteins), but if one treats it with respect, understands its characteristics and uses the usual PPE,
it is safe to handle. I can think of far, far more toxic chemicals that are tricky to handle without adopting special measures. HCN, HF or fluorine
gas to name a few (I would not touch such chemicals with a bargepole, even if it were possible for me to obtain them!). Thankfully, phenol is not too
difficult to synthesise.
As for NaF- they have no qualms putting it in toothpaste!
[Edited on 28-11-2020 by HydrogenSulphate]
|
|
|
j_sum1
Administrator
       
Posts: 6335
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
My willingness to handle dangerous chemicals has more to do wiyh my knowledge and experience than anything else.
I am ok with chlorine, bromine and NOx. And that is without a fume hood. But I know how to control production rate, scrub effectively, and pack down
without releasing uncontrolled amounts of gas.
Similarly, H2S does not strike fear into me. But I am extremely reluctant to handle HCN and cyanides in spite of HCN having roughly the same toxicity.
That is simply because I know a lot more about H2S and have some experience with its properties.
Oral ingestion is easy to mitigate against. It is the surprises that come from inhalation that worry me more. So, I really don't have issues with
Cr(VI). That said, I am usually using very small amounts. I think quantity control is the most overlooked safety measure in all OHS literature.
I do have Hg, As compounds and some Tl. I have not touched them and may not for some time. This is from lack of knowledge. I don't know the full
spectrum of properties and risks. Therefore, I don't feel equipped to work with them.
So, that's where the line is for me. More related to my knowledge than to the materials themselves.
(And yes, I have been caught out a couple of times. Greater understanding is a good thing.)
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Quantity control. Pressing words. When scaling up reactions, stuff can happen, and when it gets out of hand, it's a totally different thing when it
happens on 10mL scale than on 10 000mL scale.
Individual control on stuff is more of common safety and then a security matter. There are stupid people who mishandle the stuff and cause accidents
to themselves and others, and then there are bad people who intent to cause accidents to others. For the motivated, most things can be circumvented
somewhat easily, though, but restrictions appears to have effect on idiots.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
I very much agree with j_sum1's comments.
I try to think ahead a bit more and avoid chemicals I am not comfortable with. I have small quantities of mercury salts but I do not intend to do
anything with them. I was at one point keen on making arsenic triiodide, but will avoid arcenic for now. Also not keen on thallium. I have some uranyl
acetate, and like the idea of making uranium salts, but I am not ready for it.
I avoid cyanides. I dont want to make bromine as I dont know how to store it (never made ampoules).
I am respectful of H2S and NOx fumes but not scared - these are smells I fondly remember from my lab when I was a teenager in the late '70s!
|
|
|
artemov
Hazard to Others
  
Posts: 181
Registered: 22-8-2018
Member Is Offline
|
|
Have made potassium dichromate, lead acetate, nickel sulfate/chloride.
Have made hydrazine -> hydrazine sulfate -> potassium azide (which I destroyed almost immediately)
Have generated chlorine from TCCA and HCl.
Wanted to make bromine for fun, but decided not to cos I have no use for it and I doubt I can store it properly.
Will not deal with toxic gases like HCN, phosgene (no fume hood) or any mercury, thallium, arsenic salts.
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Quote: Originally posted by MidLifeChemist  | I'm wondering where people draw the line regarding the most toxic chemicals they are comfortable handling in their home lab, and what, if any
additional precautions they take other than the normal PPE (goggles, gloves, lab coat).
[Edited on 11/29/2020 by MidLifeChemist] |
No matter what I am doing I always put on eye protection, a coat and nitrile gloves when I enter the lab. If I am doing anything that might generated
toxic fumes I will wear a full face respirator with appropriate cartridges.
My most toxic compounds are currently in the lab are:
Mercury and it's nitrate
Cadmium and it's nitrate
Hydrazine sulfate
Benzo alpha pyrene
There is nothing I won't work with, but I spend a lot of time researching and purchasing equipment before I proceed with a synthesis involving toxic
precursors of products.
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
These are some things I don't want to handle in my lab:
- Anything explosive is out of the question.
- Highly toxic gasses like phosgene, HCN, or gasses that would cause a major nuisance to the neighbors like H2S. NO2 or Cl2, Br2 are much less
problematic in comparison.
- Beryllium, Thallium, Mercury unless the reaction is quantitative. This is because I don't want to have waste from these metals.
- Fine powders of carcinogens. I once transfered some potassium dichromate to a new bottle and I am glad I did it outside with a dust mask because
that stuff gets everywhere!
- Lacrymatory substances like esters of chloroacetic, bromoacetic acid, benzyl chloride, etc.
|
|
|
HydrogenSulphate
Harmless

Posts: 38
Registered: 13-10-2019
Member Is Offline
Mood: Caffeinated
|
|
I strongly practice the concept of atom economy, aka recycling. As such, I don't produce disposable wastes, aside from aqueous salts of low value and
low toxicity (calcium/sodium chloride, calcium/sodium acetate, calcium/sodium sulphate, and the like) that can safely be poured down the drain.
Transition metal compounds are recycled: by applying a little bit of chemical 'wizardry' it is easy to interconvert between soluble and non-soluble
forms of a transition metal compound, and to change the oxidation state to the desired one.
Volatile aromatic and aliphatic organics are purified and recaptured by distillation and condensation. Vapours are condensed back into a liquid and
collected into a GL45. By practicing on a microscale (all of my syntheses can be comfortably accommodated in a 100mL round bottom flask) and
recapturing vapours by distillation and condensation, little volatile organics are lost to the atmosphere.
[Edited on 29-11-2020 by HydrogenSulphate]
[Edited on 29-11-2020 by HydrogenSulphate]
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
I have read- and I have no reason to doubt it- that brazil nuts have a relatively large radium content; much more than most foodstuffs.
If I had a good alpha counter I might think about buying a kilo of nuts, ashing them, reducing hell out of the ashes with sodium carbonate and
charcoal at bright red heat.
Rinse that product with water to remove sodium sulphide etc.
Then leach with acetic acid and add chromate.
That should precipitate barium chromate reasonably selectively.
It should contain a few dozen becquerels of radium.
Would that count as "working with radium in a home lab"?
Would anyone be worried about the radium, given that it was delivered as food?
My point is that , as Paracelsus pointed out, it's the dose that makes a poison.
|
|
|
itsallgoodjames
Hazard to Others
  
Posts: 276
Registered: 31-8-2020
Location: America Lite
Member Is Offline
|
|
The most toxic/carcinogenic chemicals I have and use are:
- ammonium dichromate
- potassium dichromate
- mercuric nitrate
- mercuric chloride
- uranyl nitrate
- sodium diuranate
- sodium azide
Scary, though not as bad as the rest:
- very dilute (3%)hydrofluoric acid
Nuclear physics is neat. It's a shame it's so regulated...
Now that I think about it, that's probably a good thing. Still annoying though.
|
|
|
MidLifeChemist
Hazard to Others
  
Posts: 192
Registered: 4-7-2019
Location: West Coast USA
Member Is Offline
Mood: precipitatory
|
|
@flourite - thanks for your reply. I added a link to osha.gov to my post to help with your questions.
@Lion850 - I'd like to do some Uranyl experiments. Uranyl acetate is $160 for 25grams here in the US. So maybe someday. Also - there are lots of
videos on how to make an ampule, it is fairly easy, you should try making one (without the Bromine in it).
@artemov - did you mean phosgene or phosphine? Huge difference in toxicity! (factor of 25)
@B(a)P - thanks for your reply. Out of curiosity, where is your lab, is it connected to or separate from your primary residence? It is always
interesting to see how/where people set up their labs.
@Heptylene - thanks for your reply. Did you mean phosgene or phosphine? Regarding dust, yeah I have the same issue, I have to transfer chemicals into
bottles outside with a dust mask to avoid inhalation. I have some Cr2O7 to transfer and I'm not looking forward to it.
@itsallgoodjames - thanks for the reply. Where did you get the uranium compounds from, have you done anything interesting with them yet?
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Quote: Originally posted by MidLifeChemist  | @flourite - thanks for your reply. I added a link to osha.gov to my post to help with your questions.
@B(a)P - thanks for your reply. Out of curiosity, where is your lab, is it connected to or separate from your primary residence? It is always
interesting to see how/where people set up their labs.
|
My lab is separate from my dwelling.
I also have access to a lab through work.
|
|
|
itsallgoodjames
Hazard to Others
  
Posts: 276
Registered: 31-8-2020
Location: America Lite
Member Is Offline
|
|
United nuclear. I haven't done anything interesting with them yet, I intend to turn the uranyl nitrate into a uranium and titanium* (maybe titanium,
maybe some other metal, the point is to prevent corrosion) alloy at some point, though that's pretty much at the bottom of my priority list right now.
The sodium diuranate is just a collection piece, I keep a small sample of it in the uranium spot on my element collection, until I make said uranium
metal. Uranium chemistry is neat, it's just terrifying, given you have something that has all the dangers of working with soluble mercury salts, and
it's also radioactive. That's most of the reason I haven't really done much with it.
Nuclear physics is neat. It's a shame it's so regulated...
Now that I think about it, that's probably a good thing. Still annoying though.
|
|
|
Clear_horizons_glass
Harmless

Posts: 42
Registered: 30-9-2019
Location: Pullman, WA
Member Is Offline
Mood: Glass
|
|
If I have to really clean some quartz, I use Hydroflouric Acid. But only under a fume hood with tripple gloves and a vinyl apron with lots of cover. I
only do it as a last resort.
Another rule I follow is I never heat up any glass that has residue of an unknown origin. Many people don't consider how dangerous this can be.
Clear Horizons Laboratory Glassblowing Services
-------------------------------------------------------------
www.clearhorizonsglass.com
Phone and Fax:
(855) LAB-GLAS
(855) 522-4527
Have a glass project you want made? email me at
info@clearhorizonsglass.com
or message us here |
with a U2U |
message |
\/
|
|
|
artemov
Hazard to Others
  
Posts: 181
Registered: 22-8-2018
Member Is Offline
|
|
Phosgene ...
I have a bottle of Red P stored in a box with calcium chloride dessicant. Once in a while I will open up the bottle to take a look at the Red P. There
is a strange smell though ... 
|
|
|
MidLifeChemist
Hazard to Others
  
Posts: 192
Registered: 4-7-2019
Location: West Coast USA
Member Is Offline
Mood: precipitatory
|
|
Quote: Originally posted by artemov  |
Phosgene ...
I have a bottle of Red P stored in a box with calcium chloride dessicant. Once in a while I will open up the bottle to take a look at the Red P. There
is a strange smell though ...  |
Well, if you want the smell to go away, either stop heating your Red P up with hydrogen gas, or heat all of it up with an excess of hydrogen, in a
small enclosed room. Either way, that bad smell will go away...lol
[Edited on 11/30/2020 by MidLifeChemist]
|
|
|
teodor
National Hazard
   
Posts: 924
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
I am quite comfortable with CrO3 derivatives (e.g. dichromates), V2O5, Ni, Ba, Sb, sulfides. I don't know why do you consider them as "toxic" - we
don't eat those chemicals isn't it? Pb is something which requires a bit more attention, but still is "quite manageable group" for me.
On the other hand compounds of Hg, As, Tl are really toxic from my point of view.
And I would be really scared to work with alkali cyanides. May be even too scared to work safely with them. Mostly because they really have a bad
reputation and so I have no idea what to expect from them.
So I see the most of the problem is my attitude based on those chemical properties and subjective factors. Not IDLH number.
A week ago I handled a hot nitrate salt solution with HNO3 residue. It had such kind of vapours which immediately blocks your breathing, you even
don't control it. I would say 100 ml of such hot solution can have more impact on your health than bucket of dichromate on your table.
About gases: I would definitely avoid CO. But I am quite comfortable with Cl, NO2. And I am working with H2S with some precautions.
Most toxic? I often bend FEP hoses. Near the melting point they produce some toxic vapours, I think it could contain HF. It cause very unpleasant
sensation in the throat. So, if it was HF probably it is the most toxic one. With proper ventilation/gasmask I am quite comfortable.
[Edited on 30-11-2020 by teodor]
|
|
|
itsallgoodjames
Hazard to Others
  
Posts: 276
Registered: 31-8-2020
Location: America Lite
Member Is Offline
|
|
My concern with chromates and dichromates is how carcinogenic they are. One might not even know they have inhaled some dichromate dust, and that's
concerning to me. I'm actually more comfortable working with cyanides (though not HCN), as they have mostly acute effects, so they'll give me cyanide
poisoning if I'm stupid, but they won't give me cancer 20 years down the line. Maybe a bit of an irrational fear, but heavy metal salts scare me.
Also O3 and CO are nasty. I refuse to work with ozone and carbon monoxide
[Edited on 30-11-2020 by itsallgoodjames]
Nuclear physics is neat. It's a shame it's so regulated...
Now that I think about it, that's probably a good thing. Still annoying though.
|
|
|
teodor
National Hazard
   
Posts: 924
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
Quote: Originally posted by itsallgoodjames  | | My concern with chromates and dichromates is how carcinogenic they are. One might not even know they have inhaled some dichromate dust, and that's
concerning to me. |
Are you assuming it is more carcinogenic than a smoke of a cigarette ? By the way, some relative carcinogenic scale would help with everyday
chemistry, at least which dust/vapours is better to collect first. When everything is "carcinogenic" it becomes totally misleading.
Quote: Originally posted by itsallgoodjames  |
I'm actually more comfortable working with cyanides (though not HCN), as they have mostly acute effects, so they'll give me cyanide poisoning if I'm
stupid, but they won't give me cancer 20 years down the line. Maybe a bit of an irrational fear, but heavy metal salts scare me.
|
I can work with small quantities of HCN because I have a full control of (small quantity) generation and I am using a fume hood, I know where it is
contained to neutralise it. And I never contact with it in such case. Also I can recognise its presence by smell. But any powder assume some weighing,
risk of instrument/table/whatever contamination, opening/closing manipulation of a bottle and in this case CN- scares me, it is like I have a less
control about possible cases and consequences and no ability to detect it with my senses, but probably it is just lack of experience.
I think you underestimate the acute effect, do you remember the thread of knock-out potential? I think it is much more dangerous aspect than probable
cancer. Most of cases of cancer with people I know has no roots in amateur chemistry. That means there is no direct connection of carcinogenic effect
of mild carcinogens and a cancer itself.
I never thought about O3 as a toxic substance. At least at those concentration which could be produced in a lab by some electric discharge.
[Edited on 30-11-2020 by teodor]
|
|
|
itsallgoodjames
Hazard to Others
  
Posts: 276
Registered: 31-8-2020
Location: America Lite
Member Is Offline
|
|
O3 really is quite toxic. The OSHA limit for ozone in workplaces is something like 100ppb. Not 100 parts per million, 100 parts per billion. For
reference the limit for hydrogen cyanide is 40ppm and Carbon monoxide is 50ppm. Not that OSHA limits are a great way of Ozone is remarkably toxic. I
guess it's a good thing it has such a potent odour. if you have any doubt, look at it's fire diamond:
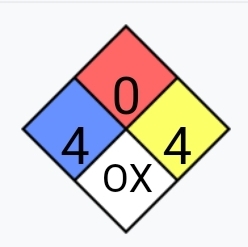
Nuclear physics is neat. It's a shame it's so regulated...
Now that I think about it, that's probably a good thing. Still annoying though.
|
|
|
teodor
National Hazard
   
Posts: 924
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
Are home ozonators (like https://www.amazon.com/customerpicks/Explore-ozone-machines-...) really intended to saturate your house with something with OSHA 100ppb?
[Edited on 30-11-2020 by teodor]
The idea of my previous post is that numbers like IDLH or OSHA could be misleading without some details including mechanism of action. If one try to
handle chemicals only according to these numbers he will present real danger for the society. I can't see any reason why HCN generator should be
considered more dangerous than O3 generator if I look only on those numbers.
As I mentioned before, according to IDLH, HBr is 2 times more dangerous than HCN.
[Edited on 30-11-2020 by teodor]
|
|
|
MidLifeChemist
Hazard to Others
  
Posts: 192
Registered: 4-7-2019
Location: West Coast USA
Member Is Offline
Mood: precipitatory
|
|
@B(a)P - you are lucky to have a separate lab, that is great. Did you ever make the WFNA?
@Clear_horizons_glass - what strength is your HF?
@Teodor -it is not my decision to call these chemicals "toxic", I am simply using the words defined by the CDC and OSHA in my country (for better or
worse). Btw, OSHA does rank all chemicals by their risks from acute and long term exposures, I looked up the values and you can see them in my
original post. For example, for C2O7, level "1A" is the highest level for a carcinogen. But I do think comparing it to cigarettes is a good question.
@itsallgoodjames - yeah, my main concern is long-term & repeated exposure from dusts and powders that I am not aware of, not eating any of the
chemicals. This may change if I can improve my clean-up procedures & air-flow.
Everyone - since we are talking about various gases like HCN, H2S, HBr, etc. - I found this very interesting page which lists IDLH
values / estimates (Immediately Dangerous to Life or Health) for a large number of compounds. Like @itsallgoodjames mentioned, the level for ozone is
much lower than other gases mentioned in this thread.
https://www.cdc.gov/niosh/idlh/intridl4.html
[Edited on 12/1/2020 by MidLifeChemist]
|
|
|
| Pages:
1
2 |